CGK733 alleviates ovariectomy-induced bone loss through blocking RANKL-mediated Ca2+ oscillations and NF-κB/MAPK signaling pathways
- PMID: 37720109
- PMCID: PMC10504545
- DOI: 10.1016/j.isci.2023.107760
CGK733 alleviates ovariectomy-induced bone loss through blocking RANKL-mediated Ca2+ oscillations and NF-κB/MAPK signaling pathways
Abstract
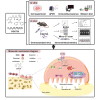
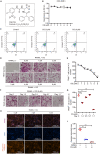
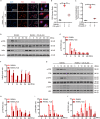
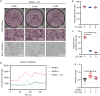
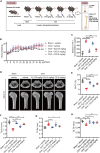
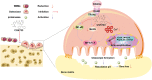
Osteoporosis is a prevalent systemic metabolic disease in modern society, in which patients often suffer from bone loss due to over-activation of osteoclasts. Currently, amelioration of bone loss through modulation of osteoclast activity is a major therapeutic strategy. Ataxia telangiectasia mutated (ATM) inhibitor CGK733 (CG) was reported to have a sensitizing impact in treating malignancies. However, its effect on osteoporosis remains unclear. In this study, we investigated the effects of CG on osteoclast differentiation and function, as well as the therapeutic effects of CG on osteoporosis. Our study found that CG inhibits osteoclast differentiation and function. We further found that CG inhibits the activation of NFATc1 and ultimately osteoclast formation by inhibiting RANKL-mediated Ca2+ oscillation and the NF-κB/MAPK signaling pathway. Next, we constructed an ovariectomized mouse model and demonstrated that CG improved bone loss in ovariectomized mice. Therefore, CG may be a potential drug for the prevention and treatment of osteoporosis.
Keywords: Biological sciences; Molecular biology; Molecular physiology.
© 2023 The Authors.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures


Similar articles
-
Maslinic acid suppresses osteoclastogenesis and prevents ovariectomy-induced bone loss by regulating RANKL-mediated NF-κB and MAPK signaling pathways.J Bone Miner Res. 2011 Mar;26(3):644-56. doi: 10.1002/jbmr.242. J Bone Miner Res. 2011. PMID: 20814972
-
Artesunate suppresses RANKL-induced osteoclastogenesis through inhibition of PLCγ1-Ca2+-NFATc1 signaling pathway and prevents ovariectomy-induced bone loss.Biochem Pharmacol. 2017 Jan 15;124:57-68. doi: 10.1016/j.bcp.2016.10.007. Epub 2016 Oct 24. Biochem Pharmacol. 2017. PMID: 27789216
-
Leonurine hydrochloride inhibits osteoclastogenesis and prevents osteoporosis associated with estrogen deficiency by inhibiting the NF-κB and PI3K/Akt signaling pathways.Bone. 2015 Jun;75:128-37. doi: 10.1016/j.bone.2015.02.017. Epub 2015 Feb 21. Bone. 2015. PMID: 25708053
-
Glaucocalyxin A suppresses osteoclastogenesis induced by RANKL and osteoporosis induced by ovariectomy by inhibiting the NF-κB and Akt pathways.J Ethnopharmacol. 2021 Aug 10;276:114176. doi: 10.1016/j.jep.2021.114176. Epub 2021 Apr 30. J Ethnopharmacol. 2021. PMID: 33933570
-
A Novel RANKL-Targeted Furoquinoline Alkaloid Ameliorates Bone Loss in Ovariectomized Osteoporosis through Inhibiting the NF-κB Signal Pathway and Reducing Reactive Oxygen Species.Oxid Med Cell Longev. 2022 Nov 4;2022:5982014. doi: 10.1155/2022/5982014. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 36388169 Free PMC article.
Cited by
-
Suppressing ERp57 diminishes osteoclast activity and ameliorates ovariectomy-induced bone loss via the intervention in calcium oscillation and the calmodulin/calcineurin/Nfatc1 pathway.Heliyon. 2024 Jul 27;10(15):e35374. doi: 10.1016/j.heliyon.2024.e35374. eCollection 2024 Aug 15. Heliyon. 2024. PMID: 39170388 Free PMC article.
-
The SRC/NF-κB-AKT/NOS3 axis as a key mediator of Kaempferol's protective effects against oxidative stress-induced osteoclastogenesis.Immun Inflamm Dis. 2024 Oct;12(10):e70045. doi: 10.1002/iid3.70045. Immun Inflamm Dis. 2024. PMID: 39422344 Free PMC article.
References
-
- Zhang Z., Wen H., Yang X., Zhang K., He B., Zhang X., Kong L. Stimuli and Relevant Signaling Cascades for NFATc1 in Bone Cell Homeostasis: Friend or Foe? Curr. Stem Cell Res. Ther. 2019;14:239–243. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

