Assessment of Financial Relationships Between Otorhinolaryngologists and Pharmaceutical Companies in Japan Between 2016 and 2019
- PMID: 37719565
- PMCID: PMC10503947
- DOI: 10.7759/cureus.43633
Assessment of Financial Relationships Between Otorhinolaryngologists and Pharmaceutical Companies in Japan Between 2016 and 2019
Abstract
Introduction: There are prevalent financial relationships between physicians and the pharmaceutical industry in medical specialties, including otorhinolaryngology. Although these relationships might cause conflicts of interest, no studies have assessed the size and contents of the financial relationships between otorhinolaryngologists and pharmaceutical companies in Japan. This study aims to evaluate the magnitude, prevalence, and trend of the financial relationship between Japanese otolaryngologists and pharmaceutical companies.
Methods: Using payment data publicly disclosed by 92 pharmaceutical companies, we examined the size, prevalence, and trend in personal payments made to the otorhinolaryngologist board certified by the Japanese Society of Otorhinolaryngology-Head and Neck Surgery (JSO-HNS) between 2016 and 2019 in Japan. Furthermore, differences in payments were evaluated by whether otolaryngologists were clinical practice guideline authors, society board members, and academic journal editors or not. Trends in payments were evaluated by generalized estimating equations.
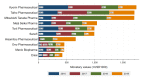
Results: Of 8,190 otorhinolaryngologists, 3,667 (44.8%) were paid a total of $13,873,562, in payments for lecturing, consulting, and writing by 72 pharmaceutical companies between 2016 and 2019. The median four-year combined payment per physician was $1,022 (interquartile range: $473-$2,526). Top 1%, 5%, and 10% of otorhinolaryngologists received 42.3% (95% confidence interval (95% CI): 37.2%-47.4%), 69.3% (95% CI: 65.9%-72.8%), and 80.6% (95% CI: 78.3%-82.9%) of overall payments, respectively. The median payments per physician were significantly higher among otorhinolaryngologists authoring clinical practice guidelines ($11,522), society board members ($22,261), and journal editors ($35,143) than those without. The payments and number of otorhinolaryngologists receiving payments remained stable between 2016 and 2019.
Conclusion: This study demonstrates that a minority but a large number of otorhinolaryngologists received personal payments from pharmaceutical companies for the reimbursement of lecturing, consulting, and writing in Japan. Large amounts of these personal payments were significantly concentrated on a small number of leading otorhinolaryngologists.
Keywords: conflict of interest; ethics; ethics and professionalism; financial conflicts of interest; health policy and economics; industry payment; japan; medical ethics; otolaryngologist; public health policy.
Copyright © 2023, Kamamoto et al.
Conflict of interest statement
The authors have declared financial relationships, which are detailed in the next section.
Figures
Similar articles
-
Financial Relationships Between Pharmaceutical Companies and Rheumatologists in Japan Between 2016 and 2019.J Clin Rheumatol. 2023 Apr 1;29(3):118-125. doi: 10.1097/RHU.0000000000001922. Epub 2022 Dec 7. J Clin Rheumatol. 2023. PMID: 36729793
-
Nature and Trends in Personal Payments Made to the Respiratory Physicians by Pharmaceutical Companies in Japan between 2016 and 2019.Respiration. 2022;101(12):1088-1098. doi: 10.1159/000526576. Epub 2022 Nov 9. Respiration. 2022. PMID: 36353778 Free PMC article.
-
Cross-sectional analysis of pharmaceutical payments to Japanese board-certified gastroenterologists between 2016 and 2019.BMJ Open. 2023 Apr 18;13(4):e068237. doi: 10.1136/bmjopen-2022-068237. BMJ Open. 2023. PMID: 37072354 Free PMC article.
-
Characteristics and Distribution of Scholarship Donations From Pharmaceutical Companies to Japanese Healthcare Institutions in 2017: A Cross-sectional Analysis.Int J Health Policy Manag. 2023;12:7621. doi: 10.34172/ijhpm.2023.7621. Epub 2023 Aug 21. Int J Health Policy Manag. 2023. PMID: 38618821 Free PMC article. Review.
-
Industry Payments to Adult Reconstruction-Trained Orthopedic Surgeons: An Analysis of the Open Payments Database From 2014 to 2019.J Arthroplasty. 2021 Nov;36(11):3788-3795. doi: 10.1016/j.arth.2021.07.004. Epub 2021 Jul 15. J Arthroplasty. 2021. PMID: 34362596 Review.
Cited by
-
Cross-sectional analysis of financial relationships between board certified allergists and the pharmaceutical industry in Japan.BMC Med Ethics. 2024 Feb 20;25(1):22. doi: 10.1186/s12910-024-01014-2. BMC Med Ethics. 2024. PMID: 38378633 Free PMC article.
-
Financial conflicts of interest among authors of clinical practice guideline for headache disorders in Japan.BMC Med Ethics. 2024 Oct 29;25(1):121. doi: 10.1186/s12910-024-01126-9. BMC Med Ethics. 2024. PMID: 39472840 Free PMC article.
-
Cross-sectional analysis of pharmaceutical industry payments to authors of clinical practice guidelines for bipolar disorder and major depressive disorder in Japan.BMJ Open. 2024 Jun 21;14(6):e086396. doi: 10.1136/bmjopen-2024-086396. BMJ Open. 2024. PMID: 38908845 Free PMC article.
-
Conflicts of Interest Among Cardiology Clinical Practice Guideline Authors in Japan.J Am Heart Assoc. 2024 Apr 16;13(8):e034506. doi: 10.1161/JAHA.124.034506. Epub 2024 Apr 12. J Am Heart Assoc. 2024. PMID: 38606773 Free PMC article.
References
-
- Data book 2023. [ Aug; 2023 ]. 2023. https://www.jpma.or.jp/news_room/issue/databook/en/rfcmr00000000an3-att/... https://www.jpma.or.jp/news_room/issue/databook/en/rfcmr00000000an3-att/...
-
- Overview and transparency of non-research payments to healthcare organizations and healthcare professionals from pharmaceutical companies in Japan: analysis of payment data in 2016. Ozaki A, Saito H, Senoo Y, et al. Health Policy. 2020;124:727–735. - PubMed
-
- Regarding the transparency guideline for the relation between corporate activities and medical institutions [Article in Japanese] [ Mar; 2022 ]. 2018. https://www.jpma.or.jp/english/code/transparency_guideline/eki4g60000003... https://www.jpma.or.jp/english/code/transparency_guideline/eki4g60000003...
LinkOut - more resources
Full Text Sources