Characterization of intracellular membrane structures derived from a massive expansion of endoplasmic reticulum (ER) membrane due to synthetic ER-membrane-resident polyproteins
- PMID: 37715992
- PMCID: PMC10735356
- DOI: 10.1093/jxb/erad364
Characterization of intracellular membrane structures derived from a massive expansion of endoplasmic reticulum (ER) membrane due to synthetic ER-membrane-resident polyproteins
Abstract
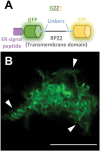
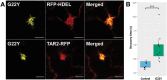
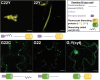
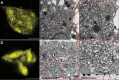
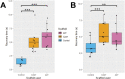
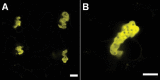
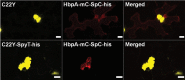
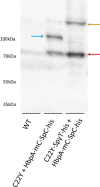
The endoplasmic reticulum (ER) is a dynamic organelle that is amenable to major restructuring. Introduction of recombinant ER-membrane-resident proteins that form homo oligomers is a known method of inducing ER proliferation: interaction of the proteins with each other alters the local structure of the ER network, leading to the formation large aggregations of expanded ER, sometimes leading to the formation of organized smooth endoplasmic reticulum (OSER). However, these membrane structures formed by ER proliferation are poorly characterized and this hampers their potential development for plant synthetic biology. Here, we characterize a range of ER-derived membranous compartments in tobacco and show how the nature of the polyproteins introduced into the ER membrane affect the morphology of the final compartment. We show that a cytosol-facing oligomerization domain is an essential component for compartment formation. Using fluorescence recovery after photobleaching, we demonstrate that although the compartment retains a connection to the ER, a diffusional barrier exists to both the ER and the cytosol associated with the compartment. Using quantitative image analysis, we also show that the presence of the compartment does not disrupt the rest of the ER network. Moreover, we demonstrate that it is possible to recruit a heterologous, bacterial enzyme to the compartment, and for the enzyme to accumulate to high levels. Finally, transgenic Arabidopsis constitutively expressing the compartment-forming polyproteins grew and developed normally under standard conditions.
Keywords: Compartment; OSER; endoplasmic reticulum; membrane; proliferation; synthetic biology.
© The Author(s) 2023. Published by Oxford University Press on behalf of the Society for Experimental Biology.
Conflict of interest statement
No conflict of interest declared.
Figures
Similar articles
-
Formation of stacked ER cisternae by low affinity protein interactions.J Cell Biol. 2003 Oct 27;163(2):257-69. doi: 10.1083/jcb.200306020. J Cell Biol. 2003. PMID: 14581454 Free PMC article.
-
Loose Morphology and High Dynamism of OSER Structures Induced by the Membrane Domain of HMG-CoA Reductase.Int J Mol Sci. 2021 Aug 24;22(17):9132. doi: 10.3390/ijms22179132. Int J Mol Sci. 2021. PMID: 34502042 Free PMC article.
-
Proliferation and Morphogenesis of the Endoplasmic Reticulum Driven by the Membrane Domain of 3-Hydroxy-3-Methylglutaryl Coenzyme A Reductase in Plant Cells.Plant Physiol. 2015 Jul;168(3):899-914. doi: 10.1104/pp.15.00597. Epub 2015 May 26. Plant Physiol. 2015. PMID: 26015445 Free PMC article.
-
Endoplasmic reticulum: a metabolic compartment.FEBS Lett. 2006 Apr 17;580(9):2160-5. doi: 10.1016/j.febslet.2006.03.050. Epub 2006 Mar 29. FEBS Lett. 2006. PMID: 16580671 Review.
-
Molecular basis for sculpting the endoplasmic reticulum membrane.Int J Biochem Cell Biol. 2012 Sep;44(9):1436-43. doi: 10.1016/j.biocel.2012.05.013. Epub 2012 May 26. Int J Biochem Cell Biol. 2012. PMID: 22640864 Review.
References
-
- Arendt P, Miettinen K, Pollier J, De Rycke R, Callewaert N, Goossens A.. 2017. An endoplasmic reticulum-engineered yeast platform for overproduction of triterpenoids. Metabolic Engineering 40, 165–175. - PubMed
-
- Barbante A, Irons S, Hawes C, Frigerio L, Vitale A, Pedrazzini E.. 2008. Anchorage to the cytosolic face of the endoplasmic reticulum membrane: a new strategy to stabilize a cytosolic recombinant antigen in plants. Plant Biotechnology Journal 6, 560–575. - PubMed
-
- Barlowe C, Helenius A.. 2016. Cargo capture and bulk flow in the early secretory pathway. Annual Review of Cell and Developmental Biology 32, 197–222. - PubMed
-
- Bolte S, Cordelières FP.. 2006. A guided tour into subcellular colocalization analysis in light microscopy. Journal of Microscopy 224, 213–232. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

