Pro-phagocytic function and structural basis of GPR84 signaling
- PMID: 37709767
- PMCID: PMC10502086
- DOI: 10.1038/s41467-023-41201-0
Pro-phagocytic function and structural basis of GPR84 signaling
Abstract
GPR84 is a unique orphan G protein-coupled receptor (GPCR) that can be activated by endogenous medium-chain fatty acids (MCFAs). The signaling of GPR84 is largely pro-inflammatory, which can augment inflammatory response, and GPR84 also functions as a pro-phagocytic receptor to enhance phagocytic activities of macrophages. In this study, we show that the activation of GPR84 by the synthetic agonist 6-OAU can synergize with the blockade of CD47 on cancer cells to induce phagocytosis of cancer cells by macrophages. We also determine a high-resolution structure of the GPR84-Gi signaling complex with 6-OAU. This structure reveals an occluded binding pocket for 6-OAU, the molecular basis of receptor activation involving non-conserved structural motifs of GPR84, and an unusual Gi-coupling interface. Together with computational docking and simulations studies, this structure also suggests a mechanism for the high selectivity of GPR84 for MCFAs and a potential routes of ligand binding and dissociation. These results provide a framework for understanding GPR84 signaling and developing new drugs targeting GPR84.
© 2023. Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures




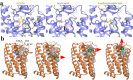

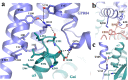
Update of
-
Pro-phagocytic function and structural basis of GPR84 signaling.Res Sq [Preprint]. 2023 Feb 15:rs.3.rs-2535247. doi: 10.21203/rs.3.rs-2535247/v1. Res Sq. 2023. Update in: Nat Commun. 2023 Sep 14;14(1):5706. doi: 10.1038/s41467-023-41201-0. PMID: 36824923 Free PMC article. Updated. Preprint.
Similar articles
-
Pro-phagocytic function and structural basis of GPR84 signaling.Res Sq [Preprint]. 2023 Feb 15:rs.3.rs-2535247. doi: 10.21203/rs.3.rs-2535247/v1. Res Sq. 2023. Update in: Nat Commun. 2023 Sep 14;14(1):5706. doi: 10.1038/s41467-023-41201-0. PMID: 36824923 Free PMC article. Updated. Preprint.
-
Agonists for G-protein-coupled receptor 84 (GPR84) alter cellular morphology and motility but do not induce pro-inflammatory responses in microglia.J Neuroinflammation. 2017 Oct 3;14(1):198. doi: 10.1186/s12974-017-0970-y. J Neuroinflammation. 2017. PMID: 28974234 Free PMC article.
-
20 Years an Orphan: Is GPR84 a Plausible Medium-Chain Fatty Acid-Sensing Receptor?DNA Cell Biol. 2020 Nov;39(11):1926-1937. doi: 10.1089/dna.2020.5846. Epub 2020 Oct 1. DNA Cell Biol. 2020. PMID: 33001759 Review.
-
Medium-chain fatty acid-sensing receptor, GPR84, is a proinflammatory receptor.J Biol Chem. 2013 Apr 12;288(15):10684-91. doi: 10.1074/jbc.M112.420042. Epub 2013 Feb 28. J Biol Chem. 2013. PMID: 23449982 Free PMC article.
-
Biased agonists of GPR84 and insights into biological control.Br J Pharmacol. 2024 May;181(10):1509-1523. doi: 10.1111/bph.16310. Epub 2024 Jan 23. Br J Pharmacol. 2024. PMID: 38148720 Review.
Cited by
-
Development of Highly Potent, G-Protein Pathway Biased, Selective, and Orally Bioavailable GPR84 Agonists.J Med Chem. 2024 Jan 11;67(1):110-137. doi: 10.1021/acs.jmedchem.3c00951. Epub 2023 Dec 26. J Med Chem. 2024. PMID: 38146625 Free PMC article.
-
Orphan GPCRs in Neurodegenerative Disorders: Integrating Structural Biology and Drug Discovery Approaches.Curr Issues Mol Biol. 2024 Oct 19;46(10):11646-11664. doi: 10.3390/cimb46100691. Curr Issues Mol Biol. 2024. PMID: 39451571 Free PMC article. Review.
-
MDSCs-derived GPR84 induces CD8+ T-cell senescence via p53 activation to suppress the antitumor response.J Immunother Cancer. 2023 Nov 28;11(11):e007802. doi: 10.1136/jitc-2023-007802. J Immunother Cancer. 2023. PMID: 38016719 Free PMC article.
-
Signal regulatory protein beta 2 is a novel positive regulator of innate anticancer immunity.Front Immunol. 2023 Dec 5;14:1287256. doi: 10.3389/fimmu.2023.1287256. eCollection 2023. Front Immunol. 2023. PMID: 38116002 Free PMC article.
References
-
- Stoddart LA, Smith NJ, Milligan G. International union of pharmacology. LXXI. Free fatty acid receptors FFA1, −2, and −3: pharmacology and pathophysiological functions. Pharmacol. Rev. 2008;60:405–417. - PubMed
-
- Kimura I, Ichimura A, Ohue-Kitano R, Igarashi M. Free fatty acid receptors in health and disease. Physiol. Rev. 2020;100:171–210. - PubMed
-
- Bergman RN, Ader M. Free fatty acids and pathogenesis of type 2 diabetes mellitus. Trends Endocrinol. Metab. 2000;11:351–356. - PubMed
-
- Alvarez-Curto E, Milligan G. Metabolism meets immunity: the role of free fatty acid receptors in the immune system. Biochem. Pharmacol. 2016;114:3–13. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

