Mice lacking γENaC palmitoylation sites maintain benzamil-sensitive Na+ transport despite reduced channel activity
- PMID: 37707951
- PMCID: PMC10721255
- DOI: 10.1172/jci.insight.172051
Mice lacking γENaC palmitoylation sites maintain benzamil-sensitive Na+ transport despite reduced channel activity
Abstract
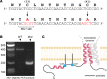
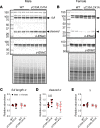
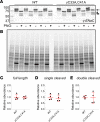
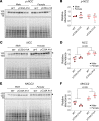
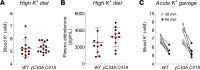
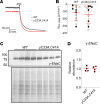
Epithelial Na+ channels (ENaCs) control extracellular fluid volume by facilitating Na+ absorption across transporting epithelia. In vitro studies showed that Cys-palmitoylation of the γENaC subunit is a major regulator of channel activity. We tested whether γ subunit palmitoylation sites are necessary for channel function in vivo by generating mice lacking the palmitoylated cysteines (γC33A,C41A) using CRISPR/Cas9 technology. ENaCs in dissected kidney tubules from γC33A,C41A mice had reduced open probability compared with wild-type (WT) littermates maintained on either standard or Na+-deficient diets. Male mutant mice also had higher aldosterone levels than WT littermates following Na+ restriction. However, γC33A,C41A mice did not have reduced amiloride-sensitive Na+ currents in the distal colon or benzamil-induced natriuresis compared to WT mice. We identified a second, larger conductance cation channel in the distal nephron with biophysical properties distinct from ENaC. The activity of this channel was higher in Na+-restricted γC33A,C41A versus WT mice and was blocked by benzamil, providing a possible compensatory mechanism for reduced prototypic ENaC function. We conclude that γ subunit palmitoylation sites are required for prototypic ENaC activity in vivo but are not necessary for amiloride/benzamil-sensitive Na+ transport in the distal nephron or colon.
Keywords: Cell Biology; Epithelial transport of ions and water; Ion channels; Nephrology.
Conflict of interest statement
Figures






Similar articles
-
Loss of the alpha subunit distal furin cleavage site blunts ENaC activation following Na+ restriction.J Physiol. 2024 Sep;602(17):4309-4326. doi: 10.1113/JP286559. Epub 2024 Aug 28. J Physiol. 2024. PMID: 39196791
-
Cysteine palmitoylation of the γ subunit has a dominant role in modulating activity of the epithelial sodium channel.J Biol Chem. 2014 May 16;289(20):14351-9. doi: 10.1074/jbc.M113.526020. Epub 2014 Apr 1. J Biol Chem. 2014. PMID: 24692558 Free PMC article.
-
Specific Palmitoyltransferases Associate with and Activate the Epithelial Sodium Channel.J Biol Chem. 2017 Mar 10;292(10):4152-4163. doi: 10.1074/jbc.M117.776146. Epub 2017 Jan 30. J Biol Chem. 2017. PMID: 28154191 Free PMC article.
-
Epithelial Na+ Channel Regulation by Extracellular and Intracellular Factors.Annu Rev Physiol. 2018 Feb 10;80:263-281. doi: 10.1146/annurev-physiol-021317-121143. Epub 2017 Nov 9. Annu Rev Physiol. 2018. PMID: 29120692 Free PMC article. Review.
-
The epithelial sodium channel and the control of sodium balance.Biochim Biophys Acta. 2010 Dec;1802(12):1159-65. doi: 10.1016/j.bbadis.2010.06.014. Epub 2010 Jun 27. Biochim Biophys Acta. 2010. PMID: 20600867 Review.
Cited by
-
Epithelial Na + Channel Activation after Bile Duct Ligation with Mineralocorticoid Receptor Blockade.J Am Soc Nephrol. 2024 Nov 1;35(11):1466-1477. doi: 10.1681/ASN.0000000000000442. Epub 2024 Jul 10. J Am Soc Nephrol. 2024. PMID: 38986682
-
Epithelial Na + Channels Function as Extracellular Sensors.Compr Physiol. 2024 Mar 29;14(2):1-41. doi: 10.1002/cphy.c230015. Compr Physiol. 2024. PMID: 39109974 Review.
-
Loss of the alpha subunit distal furin cleavage site blunts ENaC activation following Na+ restriction.J Physiol. 2024 Sep;602(17):4309-4326. doi: 10.1113/JP286559. Epub 2024 Aug 28. J Physiol. 2024. PMID: 39196791
-
Influence of proteolytic cleavage of ENaC's γ subunit upon Na+ and K+ handling.Am J Physiol Renal Physiol. 2024 Jun 1;326(6):F1066-F1077. doi: 10.1152/ajprenal.00027.2024. Epub 2024 Apr 18. Am J Physiol Renal Physiol. 2024. PMID: 38634134
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

