Ramucirumab plus FOLFIRI as second-line treatment for patients with RAS wild-type metastatic colorectal cancer previously treated with anti-EGFR antibody: JACCRO CC-16
- PMID: 37703596
- PMCID: PMC10594013
- DOI: 10.1016/j.esmoop.2023.101636
Ramucirumab plus FOLFIRI as second-line treatment for patients with RAS wild-type metastatic colorectal cancer previously treated with anti-EGFR antibody: JACCRO CC-16
Abstract
Background: Chemotherapy in combination with anti-epidermal growth factor receptor (EGFR) antibody is considered a first-line treatment regimen for RAS wild-type and left-sided metastatic colorectal cancer (mCRC), whereas second-line treatment regimens have not yet been established. Few studies have prospectively evaluated second-line treatment with anti-vascular endothelial growth factor antibody after first-line anti-EGFR antibody therapy for RAS wild-type mCRC.
Patients and methods: This non-randomized phase II trial investigated the clinical outcomes of second-line ramucirumab (RAM) plus fluorouracil, levofolinate, and irinotecan (FOLFIRI) after first-line anti-EGFR antibody in combination with doublet or triplet regimen in patients with RAS wild-type mCRC. The primary endpoint was the 6-month progression-free survival (PFS) rate. The secondary endpoints were PFS, overall survival (OS), objective response rate (ORR), rate of early tumor shrinkage (ETS), and safety. We hypothesized a threshold 6-month PFS rate of 30% and an expected 6-month PFS rate of 45%. Treatment was considered effective if the lower limit of the 90% confidence interval (CI) of the 6-month PFS rate was >0.30.
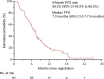
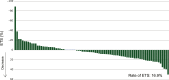
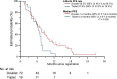
Results: Ninety-two patients were enrolled in the study. The primary tumor was located on the left side in 86 (95.6%) patients. Twenty (22.0%) patients had received triplet plus cetuximab as previous therapy. Six-month PFS rate was 58.2% (90% CI 49.3% to 66.2%) with a median PFS of 7.0 months (95% CI 5.7-7.6 months). Median OS was 23.6 months (95% CI 16.5-26.3 months). The ORR and ETS rate were 10.7% and 16.9%, respectively, in 83 patients with measurable lesions. The 6-month PFS rate was comparable between patients previously treated with doublet and triplet regimens; however, median PFS was longer for the doublet regimen (7.4 versus 6.4 months, P = 0.036).
Conclusions: Our study demonstrated prospectively that RAM plus FOLFIRI is an effective second-line treatment after anti-EGFR antibody-containing first-line therapy in RAS wild-type and left-sided mCRC. Furthermore, the results were similar for patients who were previously treated with triplet regimen.
Keywords: RAS wild-type; chemotherapy; colorectal cancer; ramucirumab.
Copyright © 2023 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Figures
Similar articles
-
FOLFOX plus anti-epidermal growth factor receptor (EGFR) monoclonal antibody (mAb) is an effective first-line treatment for patients with RAS-wild left-sided metastatic colorectal cancer: A meta-analysis.Medicine (Baltimore). 2018 Mar;97(10):e0097. doi: 10.1097/MD.0000000000010097. Medicine (Baltimore). 2018. PMID: 29517682 Free PMC article. Review.
-
Prognostic and predictive value of primary tumour side in patients with RAS wild-type metastatic colorectal cancer treated with chemotherapy and EGFR directed antibodies in six randomized trials.Ann Oncol. 2017 Aug 1;28(8):1713-1729. doi: 10.1093/annonc/mdx175. Ann Oncol. 2017. PMID: 28407110 Free PMC article.
-
Efficacy, safety and genomic analysis of SCT200, an anti-EGFR monoclonal antibody, in patients with fluorouracil, irinotecan and oxaliplatin refractory RAS and BRAF wild-type metastatic colorectal cancer: a phase Ⅱ study.EBioMedicine. 2024 Feb;100:104966. doi: 10.1016/j.ebiom.2024.104966. Epub 2024 Jan 13. EBioMedicine. 2024. PMID: 38217945 Free PMC article. Clinical Trial.
-
Clinicians' Attitude to Doublet Plus Anti-EGFR Versus Triplet Plus Bevacizumab as First-line Treatment in Left-Sided RAS and BRAF Wild-Type Metastatic Colorectal Cancer Patients: A Multicenter, "Real-Life", Case-Control Study.Clin Colorectal Cancer. 2021 Dec;20(4):318-325. doi: 10.1016/j.clcc.2021.07.003. Epub 2021 Jul 18. Clin Colorectal Cancer. 2021. PMID: 34380594
-
A meta-analysis of efficacy and safety data from head-to-head first-line trials of epidermal growth factor receptor inhibitors versus bevacizumab in adult patients with RAS wild-type metastatic colorectal cancer by sidedness.Eur J Cancer. 2024 May;202:113975. doi: 10.1016/j.ejca.2024.113975. Epub 2024 Mar 1. Eur J Cancer. 2024. PMID: 38442645 Review.
Cited by
-
Evaluation of the efficacy and safety of ramucirumab combined with nab-paclitaxel, lobaplatin, and S-1 in neoadjuvant and conversion therapy for advanced gastric cancer: A study protocol of prospective single-center, randomized controlled and open label clinical trial (RNPLS-01).Heliyon. 2024 Apr 9;10(8):e29485. doi: 10.1016/j.heliyon.2024.e29485. eCollection 2024 Apr 30. Heliyon. 2024. PMID: 38660276 Free PMC article.
References
-
- Sung H., Ferlay J., Siegel R.L., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. - PubMed
-
- Tabernero J., Yoshino T., Cohn A.L., et al. Ramucirumab versus placebo in combination with second-line FOLFIRI in patients with metastatic colorectal carcinoma that progressed during or after first-line therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine (RAISE): a randomised, double-blind, multicentre, phase 3 study. Lancet Oncol. 2015;16(5):499–508. - PubMed
-
- Van Cutsem E., Tabernero J., Lakomy R., et al. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J Clin Oncol. 2012;30(28):3499–3506. - PubMed
-
- Bennouna J., Sastre J., Arnold D., et al. Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): a randomised phase 3 trial. Lancet Oncol. 2013;14(1):29–37. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

