A new strategy for treating colorectal cancer: Regulating the influence of intestinal flora and oncolytic virus on interferon
- PMID: 37701850
- PMCID: PMC10493895
- DOI: 10.1016/j.omto.2023.08.010
A new strategy for treating colorectal cancer: Regulating the influence of intestinal flora and oncolytic virus on interferon
Abstract
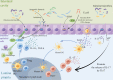
Colorectal cancer (CRC) has the third highest incidence and the second highest mortality in the world, which seriously affects human health, while current treatments methods for CRC, including systemic therapy, preoperative radiotherapy, and surgical local excision, still have poor survival rates for patients with metastatic disease, making it critical to develop new strategies for treating CRC. In this article, we found that the gut microbiota can modulate the signaling pathways of cancer cells through direct contact with tumor cells, generate inflammatory responses and oxidative stress through interactions between the innate and adaptive immune systems, and produce diverse metabolic combinations to trigger specific immune responses and promote the initiation of systemic type I interferon (IFN-I) and anti-viral immunity. In addition, oncolytic virus-mediated immunotherapy for regulating oncolytic virus can directly lyse tumor cells, induce the immune activity of the body, interact with interferon, inhibit the anti-viral effect of IFN-I, and enhance the anti-tumor effect of IFN-II. Interferon plays an important role in the anti-tumor process. We put forward that exploring the effects of intestinal flora and oncolytic virus on interferon to treat CRC is a promising therapeutic option.
Keywords: basic mechanism; colorectal cancer; interaction; interferon; intestinal flora; oncolytic virus.
© 2023 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
The γδ T cells dual function and crosstalk with intestinal flora in treating colorectal cancer is a promising area of study.Int Immunopharmacol. 2023 Oct;123:110733. doi: 10.1016/j.intimp.2023.110733. Epub 2023 Aug 12. Int Immunopharmacol. 2023. PMID: 37579540 Review.
-
Gut microbiota interactions with antitumor immunity in colorectal cancer: From understanding to application.Biomed Pharmacother. 2023 Sep;165:115040. doi: 10.1016/j.biopha.2023.115040. Epub 2023 Jun 24. Biomed Pharmacother. 2023. PMID: 37364479 Review.
-
Exploring the Interactions of Oncolytic Viral Therapy and Immunotherapy of Anti-CTLA-4 for Malignant Melanoma Mice Model.Cells. 2023 Feb 3;12(3):507. doi: 10.3390/cells12030507. Cells. 2023. PMID: 36766849 Free PMC article.
-
Engineered Oncolytic Poliovirus PVSRIPO Subverts MDA5-Dependent Innate Immune Responses in Cancer Cells.J Virol. 2018 Sep 12;92(19):e00879-18. doi: 10.1128/JVI.00879-18. Print 2018 Oct 1. J Virol. 2018. PMID: 29997212 Free PMC article.
-
The Intestinal Microbiome Primes Host Innate Immunity against Enteric Virus Systemic Infection through Type I Interferon.mBio. 2021 May 11;12(3):e00366-21. doi: 10.1128/mBio.00366-21. mBio. 2021. PMID: 33975932 Free PMC article.
Cited by
-
Gut microbiota reshapes cancer immunotherapy efficacy: Mechanisms and therapeutic strategies.Imeta. 2024 Jan 1;3(1):e156. doi: 10.1002/imt2.156. eCollection 2024 Feb. Imeta. 2024. PMID: 38868510 Free PMC article. Review.
References
-
- Dunn G.P., Koebel C.M., Schreiber R.D. Interferons, immunity and cancer immunoediting. Nat. Rev. Immunol. 2006;6:836–848. - PubMed
-
- Takaoka A., Hayakawa S., Yanai H., Stoiber D., Negishi H., Kikuchi H., Sasaki S., Imai K., Shibue T., Honda K., Taniguchi T. Integration of interferon-alpha/beta signalling to p53 responses in tumour suppression and antiviral defence. Nature. 2003;424:516–523. - PubMed
-
- Silverman R.H. Implications for RNase L in prostate cancer biology. Biochemistry. 2003;42:1805–1812. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

