PARP12 is required to repress the replication of a Mac1 mutant coronavirus in a cell- and tissue-specific manner
- PMID: 37695054
- PMCID: PMC10537751
- DOI: 10.1128/jvi.00885-23
PARP12 is required to repress the replication of a Mac1 mutant coronavirus in a cell- and tissue-specific manner
Abstract
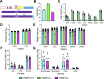
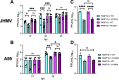
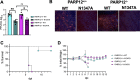
ADP-ribosyltransferases (ARTs) mediate the transfer of ADP-ribose from NAD+ to protein or nucleic acid substrates. This modification can be removed by several different types of proteins, including macrodomains. Several ARTs, also known as PARPs, are stimulated by interferon indicating ADP-ribosylation is an important aspect of the innate immune response. All coronaviruses (CoVs) encode for a highly conserved macrodomain (Mac1) that is critical for CoVs to replicate and cause disease, indicating that ADP-ribosylation can effectively control coronavirus infection. Our siRNA screen indicated that PARP12 might inhibit the replication of a murine hepatitis virus (MHV) Mac1 mutant virus in bone-marrow-derived macrophages (BMDMs). To conclusively demonstrate that PARP12 is a key mediator of the antiviral response to CoVs both in cell culture and in vivo, we produced PARP12-/-mice and tested the ability of MHV A59 (hepatotropic/neurotropic) and JHM (neurotropic) Mac1 mutant viruses to replicate and cause disease in these mice. Notably, in the absence of PARP12, Mac1 mutant replication was increased in BMDMs and mice. In addition, liver pathology was also increased in A59-infected mice. However, the PARP12 knockout did not restore Mac1 mutant virus replication to WT virus levels in all cell or tissue types and did not significantly increase the lethality of Mac1 mutant viruses. These results demonstrate that while PARP12 inhibits MHV Mac1 mutant virus infection, additional PARPs or innate immune factors must contribute to the extreme attenuation of this virus in mice. IMPORTANCE Over the last decade, the importance of ADP-ribosyltransferases (ARTs), also known as PARPs, in the antiviral response has gained increased significance as several were shown to either restrict virus replication or impact innate immune responses. However, there are few studies showing ART-mediated inhibition of virus replication or pathogenesis in animal models. We found that the CoV macrodomain (Mac1) was required to prevent ART-mediated inhibition of virus replication in cell culture. Using knockout mice, we found that PARP12, an interferon-stimulated ART, was required to repress the replication of a Mac1 mutant CoV both in cell culture and in mice, demonstrating that PARP12 represses coronavirus replication. However, the deletion of PARP12 did not fully rescue Mac1 mutant virus replication or pathogenesis, indicating that multiple PARPs function to counter coronavirus infection.
Keywords: ADP-ribosylation; MHV; PARP; coronavirus; macrodomain; pathology.
Conflict of interest statement
Anthony R. Fehr was named as an inventor on a patent filed by the University of Kansas.
Figures
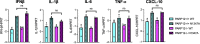


Update of
-
PARP12 is required to repress the replication of a Mac1 mutant coronavirus in a cell and tissue specific manner.bioRxiv [Preprint]. 2023 Jun 17:2023.06.16.545351. doi: 10.1101/2023.06.16.545351. bioRxiv. 2023. Update in: J Virol. 2023 Sep 28;97(9):e0088523. doi: 10.1128/jvi.00885-23 PMID: 37398292 Free PMC article. Updated. Preprint.
Similar articles
-
Mutation of a highly conserved isoleucine residue in loop 2 of several β-coronavirus macrodomains indicates that enhanced ADP-ribose binding is detrimental for replication.J Virol. 2024 Nov 19;98(11):e0131324. doi: 10.1128/jvi.01313-24. Epub 2024 Oct 10. J Virol. 2024. PMID: 39387584
-
PARP12 is required to repress the replication of a Mac1 mutant coronavirus in a cell and tissue specific manner.bioRxiv [Preprint]. 2023 Jun 17:2023.06.16.545351. doi: 10.1101/2023.06.16.545351. bioRxiv. 2023. Update in: J Virol. 2023 Sep 28;97(9):e0088523. doi: 10.1128/jvi.00885-23 PMID: 37398292 Free PMC article. Updated. Preprint.
-
Unique Mutations in the Murine Hepatitis Virus Macrodomain Differentially Attenuate Virus Replication, Indicating Multiple Roles for the Macrodomain in Coronavirus Replication.J Virol. 2021 Jul 12;95(15):e0076621. doi: 10.1128/JVI.00766-21. Epub 2021 Jul 12. J Virol. 2021. PMID: 34011547 Free PMC article.
-
The Viral Macrodomain Counters Host Antiviral ADP-Ribosylation.Viruses. 2020 Mar 31;12(4):384. doi: 10.3390/v12040384. Viruses. 2020. PMID: 32244383 Free PMC article. Review.
-
Host ADP-ribosylation and the SARS-CoV-2 macrodomain.Biochem Soc Trans. 2021 Aug 27;49(4):1711-1721. doi: 10.1042/BST20201212. Biochem Soc Trans. 2021. PMID: 34351418 Free PMC article. Review.
Cited by
-
PARP14 and PARP9/DTX3L regulate interferon-induced ADP-ribosylation.EMBO J. 2024 Jul;43(14):2929-2953. doi: 10.1038/s44318-024-00126-0. Epub 2024 Jun 4. EMBO J. 2024. PMID: 38834853 Free PMC article.
-
Mutation of a highly conserved isoleucine residue in loop 2 of several β-coronavirus macrodomains indicates that enhanced ADP-ribose binding is detrimental to infection.bioRxiv [Preprint]. 2024 Jul 12:2024.01.03.574082. doi: 10.1101/2024.01.03.574082. bioRxiv. 2024. Update in: J Virol. 2024 Nov 19;98(11):e0131324. doi: 10.1128/jvi.01313-24 PMID: 38260573 Free PMC article. Updated. Preprint.
-
Mutation of a highly conserved isoleucine residue in loop 2 of several β-coronavirus macrodomains indicates that enhanced ADP-ribose binding is detrimental for replication.J Virol. 2024 Nov 19;98(11):e0131324. doi: 10.1128/jvi.01313-24. Epub 2024 Oct 10. J Virol. 2024. PMID: 39387584
-
The Mac1 ADP-ribosylhydrolase is a Therapeutic Target for SARS-CoV-2.bioRxiv [Preprint]. 2024 Aug 29:2024.08.08.606661. doi: 10.1101/2024.08.08.606661. bioRxiv. 2024. PMID: 39149230 Free PMC article. Preprint.
-
PARP14 is regulated by the PARP9/DTX3L complex and promotes interferon γ-induced ADP-ribosylation.EMBO J. 2024 Jul;43(14):2908-2928. doi: 10.1038/s44318-024-00125-1. Epub 2024 Jun 4. EMBO J. 2024. PMID: 38834852 Free PMC article.
References
-
- Comar CE, Otter CJ, Pfannenstiel J, Doerger E, Renner DM, Tan LH, Perlman S, Cohen NA, Fehr AR, Weiss SR. 2022. MERS-CoV endoribonuclease and accessory proteins jointly evade host innate immunity during infection of lung and nasal epithelial cells. Proc Natl Acad Sci U S A 119:e2123208119. doi:10.1073/pnas.2123208119 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

