VAMP726 from maize and Arabidopsis confers pollen resistance to heat and UV radiation by influencing lignin content of sporopollenin
- PMID: 37691288
- PMCID: PMC10721520
- DOI: 10.1016/j.xplc.2023.100682
VAMP726 from maize and Arabidopsis confers pollen resistance to heat and UV radiation by influencing lignin content of sporopollenin
Abstract
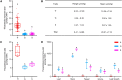
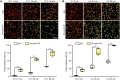
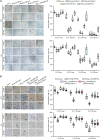
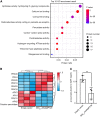
Sporopollenin in the pollen cell wall protects male gametophytes from stresses. Phenylpropanoid derivatives, including guaiacyl (G) lignin units, are known to be structural components of sporopollenin, but the exact composition of sporopollenin remains to be fully resolved. We analyzed the phenylpropanoid derivatives in sporopollenin from maize and Arabidopsis by thioacidolysis coupled with nuclear magnetic resonance (NMR) and gas chromatography-mass spectrometry (GC-MS). The NMR and GC-MS results confirmed the presence of p-hydroxyphenyl (H), G, and syringyl (S) lignin units in sporopollenin from maize and Arabidopsis. Strikingly, H units account for the majority of lignin monomers in sporopollenin from these species. We next performed a genome-wide association study to explore the genetic basis of maize sporopollenin composition and identified a vesicle-associated membrane protein (ZmVAMP726) that is strongly associated with lignin monomer composition of maize sporopollenin. Genetic manipulation of VAMP726 affected not only lignin monomer composition in sporopollenin but also pollen resistance to heat and UV radiation in maize and Arabidopsis, indicating that VAMP726 is functionally conserved in monocot and dicot plants. Our work provides new insight into the lignin monomers that serve as structural components of sporopollenin and characterizes VAMP726, which affects sporopollenin composition and stress resistance in pollen.
Keywords: UV radiation; heat stress; lignin monomers; pollen cell wall; sporopollenin.
Copyright © 2023 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures



Similar articles
-
Phenylpropanoid Derivatives Are Essential Components of Sporopollenin in Vascular Plants.Mol Plant. 2020 Nov 2;13(11):1644-1653. doi: 10.1016/j.molp.2020.08.005. Epub 2020 Aug 15. Mol Plant. 2020. PMID: 32810599
-
CRISPR/Cas9-based genome editing of 14 lipid metabolic genes reveals a sporopollenin metabolon ZmPKSB-ZmTKPR1-1/-2 required for pollen exine formation in maize.Plant Biotechnol J. 2024 Jan;22(1):216-232. doi: 10.1111/pbi.14181. Epub 2023 Oct 4. Plant Biotechnol J. 2024. PMID: 37792967 Free PMC article.
-
Probing native lignin macromolecular configuration in Arabidopsis thaliana in specific cell wall types: further insights into limited substrate degeneracy and assembly of the lignins of ref8, fah 1-2 and C4H::F5H lines.Mol Biosyst. 2010 Mar;6(3):499-515. doi: 10.1039/b819206e. Epub 2009 Dec 10. Mol Biosyst. 2010. PMID: 20174679
-
The biosynthesis, composition and assembly of the outer pollen wall: A tough case to crack.Phytochemistry. 2015 May;113:170-82. doi: 10.1016/j.phytochem.2014.05.002. Epub 2014 Jun 3. Phytochemistry. 2015. PMID: 24906292 Review.
-
Trends in lignin modification: a comprehensive analysis of the effects of genetic manipulations/mutations on lignification and vascular integrity.Phytochemistry. 2002 Oct;61(3):221-94. doi: 10.1016/s0031-9422(02)00211-x. Phytochemistry. 2002. PMID: 12359514 Review.
Cited by
-
Integrative Dissection of Lignin Composition in Tartary Buckwheat Seed Hulls for Enhanced Dehulling Efficiency.Adv Sci (Weinh). 2024 May;11(20):e2400916. doi: 10.1002/advs.202400916. Epub 2024 Mar 23. Adv Sci (Weinh). 2024. PMID: 38520733 Free PMC article.
-
Potent pollen gene regulation by DNA glycosylases in maize.Nat Commun. 2024 Sep 27;15(1):8352. doi: 10.1038/s41467-024-52620-y. Nat Commun. 2024. PMID: 39333110 Free PMC article.
References
-
- Barros J., Escamilla-Trevino L., Song L., Rao X., Serrani-Yarce J.C., Palacios M.D., Engle N., Choudhury F.K., Tschaplinski T.J., Venables B.J., et al. 4-Coumarate 3-hydroxylase in the lignin biosynthesis pathway is a cytosolic ascorbate peroxidase. Nat. Commun. 2019;10:1994. doi: 10.1038/s41467-019-10082-7. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

