Role of Leptin and Adiponectin in Carcinogenesis
- PMID: 37686525
- PMCID: PMC10486522
- DOI: 10.3390/cancers15174250
Role of Leptin and Adiponectin in Carcinogenesis
Abstract
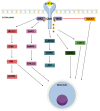
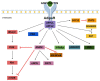
Hormones produced by adipocytes, leptin and adiponectin, are associated with the process of carcinogenesis. Both of these adipokines have well-proven oncologic potential and can affect many aspects of tumorigenesis, from initiation and primary tumor growth to metastatic progression. Involvement in the formation of cancer includes interactions with the tumor microenvironment and its components, such as tumor-associated macrophages, cancer-associated fibroblasts, extracellular matrix and matrix metalloproteinases. Furthermore, these adipokines participate in the epithelial-mesenchymal transition and connect to angiogenesis, which is critical for cancer invasiveness and cancer cell migration. In addition, an enormous amount of evidence has demonstrated that altered concentrations of these adipocyte-derived hormones and the expression of their receptors in tumors are associated with poor prognosis in various types of cancer. Therefore, leptin and adiponectin dysfunction play a prominent role in cancer and impact tumor invasion and metastasis in different ways. This review clearly and comprehensively summarizes the recent findings and presents the role of leptin and adiponectin in cancer initiation, promotion and progression, focusing on associations with the tumor microenvironment and its components as well as roles in the epithelial-mesenchymal transition and angiogenesis.
Keywords: adiponectin; angiogenesis; epithelial–mesenchymal transition; leptin; tumor microenvironment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Adipocyte-derived IL-6 and leptin promote breast Cancer metastasis via upregulation of Lysyl Hydroxylase-2 expression.Cell Commun Signal. 2018 Dec 18;16(1):100. doi: 10.1186/s12964-018-0309-z. Cell Commun Signal. 2018. PMID: 30563531 Free PMC article.
-
The potential role of leptin in tumor invasion and metastasis.Cytokine Growth Factor Rev. 2017 Dec;38:80-97. doi: 10.1016/j.cytogfr.2017.11.002. Epub 2017 Nov 11. Cytokine Growth Factor Rev. 2017. PMID: 29158066 Free PMC article. Review.
-
Adipo-oncology: adipocyte-derived factors govern engraftment, survival, and progression of metastatic cancers.Cell Commun Signal. 2024 Jan 18;22(1):52. doi: 10.1186/s12964-024-01474-4. Cell Commun Signal. 2024. PMID: 38238841 Free PMC article. Review.
-
Breast cancer and obesity: in vitro interferences between adipokines and proangiogenic features and/or antitumor therapies?PLoS One. 2013;8(3):e58541. doi: 10.1371/journal.pone.0058541. Epub 2013 Mar 15. PLoS One. 2013. PMID: 23554900 Free PMC article.
-
Adipokines and epithelial-mesenchymal transition (EMT) in cancer.Mol Cell Biochem. 2023 Nov;478(11):2419-2433. doi: 10.1007/s11010-023-04670-x. Epub 2023 Jan 30. Mol Cell Biochem. 2023. PMID: 36715963 Review.
Cited by
-
Relationship between adipocytes and hematological tumors in the bone marrow microenvironment: a literature review.Transl Cancer Res. 2024 Oct 31;13(10):5691-5701. doi: 10.21037/tcr-24-52. Epub 2024 Oct 12. Transl Cancer Res. 2024. PMID: 39525009 Free PMC article. Review.
-
Towards Understanding the Development of Breast Cancer: The Role of RhoJ in the Obesity Microenvironment.Cells. 2024 Jan 17;13(2):174. doi: 10.3390/cells13020174. Cells. 2024. PMID: 38247865 Free PMC article.
-
Cancer-associated adipocytes in the ovarian cancer microenvironment.Am J Cancer Res. 2024 Jul 15;14(7):3259-3279. doi: 10.62347/XZRI9189. eCollection 2024. Am J Cancer Res. 2024. PMID: 39113876 Free PMC article. Review.
-
Obesity and Cancer Rehabilitation for Functional Recovery and Quality of Life in Breast Cancer Survivors: A Comprehensive Review.Cancers (Basel). 2024 Jan 25;16(3):521. doi: 10.3390/cancers16030521. Cancers (Basel). 2024. PMID: 38339271 Free PMC article. Review.
-
Lung cancer and obesity: A contentious relationship (Review).Oncol Rep. 2024 Nov;52(5):158. doi: 10.3892/or.2024.8817. Epub 2024 Nov 4. Oncol Rep. 2024. PMID: 39497438 Free PMC article. Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

