FoxO3 Modulates Circadian Rhythms in Neural Stem Cells
- PMID: 37686468
- PMCID: PMC10563086
- DOI: 10.3390/ijms241713662
FoxO3 Modulates Circadian Rhythms in Neural Stem Cells
Abstract
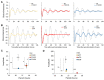
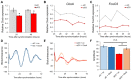
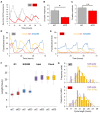
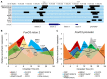
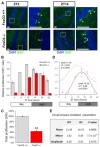
Both FoxO transcription factors and the circadian clock act on the interface of metabolism and cell cycle regulation and are important regulators of cellular stress and stem cell homeostasis. Importantly, FoxO3 preserves the adult neural stem cell population by regulating cell cycle and cellular metabolism and has been shown to regulate circadian rhythms in the liver. However, whether FoxO3 is a regulator of circadian rhythms in neural stem cells remains unknown. Here, we show that loss of FoxO3 disrupts circadian rhythmicity in cultures of neural stem cells, an effect that is mediated via regulation of Clock transcriptional levels. Using Rev-Erbα-VNP as a reporter, we then demonstrate that loss of FoxO3 does not disrupt circadian rhythmicity at the single cell level. A meta-analysis of published data revealed dynamic co-occupancy of multiple circadian clock components within FoxO3 regulatory regions, indicating that FoxO3 is a Clock-controlled gene. Finally, we examined proliferation in the hippocampus of FoxO3-deficient mice and found that loss of FoxO3 delayed the circadian phase of hippocampal proliferation, indicating that FoxO3 regulates correct timing of NSC proliferation. Taken together, our data suggest that FoxO3 is an integral part of circadian regulation of neural stem cell homeostasis.
Keywords: FoxO3; cell cycle; circadian rhythms; liver; metabolism; neural stem cell.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Insulin-FOXO3 signaling modulates circadian rhythms via regulation of clock transcription.Curr Biol. 2014 Jun 2;24(11):1248-55. doi: 10.1016/j.cub.2014.04.018. Epub 2014 May 22. Curr Biol. 2014. PMID: 24856209
-
Circadian Clock Component Rev-erbα Regulates Diurnal Rhythm of UDP-Glucuronosyltransferase 1a9 and Drug Glucuronidation in Mice.Drug Metab Dispos. 2020 Aug;48(8):681-689. doi: 10.1124/dmd.120.000030. Epub 2020 Jun 11. Drug Metab Dispos. 2020. PMID: 32527940
-
REV-ERBα alters circadian rhythms by modulating mTOR signaling.Mol Cell Endocrinol. 2021 Feb 5;521:111108. doi: 10.1016/j.mce.2020.111108. Epub 2020 Dec 5. Mol Cell Endocrinol. 2021. PMID: 33285244
-
Role of the clock gene Rev-erbα in metabolism and in the endocrine pancreas.Diabetes Obes Metab. 2015 Sep;17 Suppl 1:106-14. doi: 10.1111/dom.12522. Diabetes Obes Metab. 2015. PMID: 26332975 Review.
-
Chronopharmacological strategies focused on chrono-drug discovery.Pharmacol Ther. 2019 Oct;202:72-90. doi: 10.1016/j.pharmthera.2019.05.018. Epub 2019 Jun 5. Pharmacol Ther. 2019. PMID: 31173839 Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

