Meiotic Cell Cycle Progression in Mouse Oocytes: Role of Cyclins
- PMID: 37686466
- PMCID: PMC10487953
- DOI: 10.3390/ijms241713659
Meiotic Cell Cycle Progression in Mouse Oocytes: Role of Cyclins
Abstract
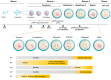
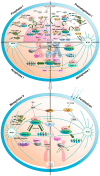
All eukaryotic cells, including oocytes, utilize an engine called cyclin-dependent kinase (Cdk) to drive the cell cycle. Cdks are activated by a co-factor called cyclin, which regulates their activity. The key Cdk-cyclin complex that regulates the oocyte cell cycle is known as Cdk1-cyclin B1. Recent studies have elucidated the roles of other cyclins, such as B2, B3, A2, and O, in oocyte cell cycle regulation. This review aims to discuss the recently discovered roles of various cyclins in mouse oocyte cell cycle regulation in accordance with the sequential progression of the cell cycle. In addition, this review addresses the translation and degradation of cyclins to modulate the activity of Cdks. Overall, the literature indicates that each cyclin performs unique and redundant functions at various stages of the cell cycle, while their expression and degradation are tightly regulated. Taken together, this review provides new insights into the regulatory role and function of cyclins in oocyte cell cycle progression.
Keywords: cyclin; maturation promoting factor; oocyte maturation; protein modification; translational regulation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Cyclins regulating oocyte meiotic cell cycle progression†.Biol Reprod. 2019 Nov 21;101(5):878-881. doi: 10.1093/biolre/ioz143. Biol Reprod. 2019. PMID: 31347666 Free PMC article. Review.
-
Temporal patterns of gene expression of G1-S cyclins and cdks during the first and second mitotic cell cycles in mouse embryos.Mol Reprod Dev. 1996 Nov;45(3):264-75. doi: 10.1002/(SICI)1098-2795(199611)45:3<264::AID-MRD2>3.0.CO;2-Q. Mol Reprod Dev. 1996. PMID: 8916036
-
Cyclins and CDKS in development and cancer: lessons from genetically modified mice.Front Biosci. 2006 Jan 1;11:1164-88. doi: 10.2741/1871. Front Biosci. 2006. PMID: 16146805 Review.
-
Cycling through mammalian meiosis: B-type cyclins in oocytes.Cell Cycle. 2019 Jul;18(14):1537-1548. doi: 10.1080/15384101.2019.1632139. Epub 2019 Jun 23. Cell Cycle. 2019. PMID: 31208271 Free PMC article.
-
Animal Models for Studying the In Vivo Functions of Cell Cycle CDKs.Methods Mol Biol. 2016;1336:155-66. doi: 10.1007/978-1-4939-2926-9_13. Methods Mol Biol. 2016. PMID: 26231715
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

