Improvement of Vascular Insulin Sensitivity by Ranolazine
- PMID: 37686345
- PMCID: PMC10487645
- DOI: 10.3390/ijms241713532
Improvement of Vascular Insulin Sensitivity by Ranolazine
Abstract
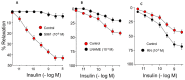
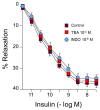
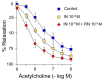
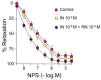
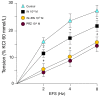
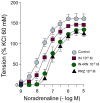
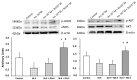
Ranolazine (RN) is a drug used in the treatment of chronic coronary ischemia. Different clinical trials have shown that RN behaves as an anti-diabetic drug by lowering blood glucose and glycosylated hemoglobin (HbA1c) levels. However, RN has not been shown to improve insulin (IN) sensitivity. Our study investigates the possible facilitating effects of RN on the actions of IN in the rabbit aorta. IN induced vasodilation of the abdominal aorta in a concentration-dependent manner, and this dilatory effect was due to the phosphorylation of endothelial nitric oxide synthase (eNOS) and the formation of nitric oxide (NO). On the other hand, IN facilitated the vasodilator effects of acetylcholine but not the vasodilation induced by sodium nitroprusside. RN facilitated all the vasodilatory effects of IN. In addition, IN decreased the vasoconstrictor effects of adrenergic nerve stimulation and exogenous noradrenaline. Both effects were in turn facilitated by RN. The joint effect of RN with IN induced a significant increase in the ratio of p-eNOS/eNOS and pAKT/AKT. In conclusion, RN facilitated the vasodilator effects of IN, both direct and induced, on the adrenergic system. Therefore, RN increases vascular sensitivity to IN, thus decreasing tissue resistance to the hormone, a key mechanism in the development of type II diabetes.
Keywords: adrenergic system; insulin; nitric oxide; ranolazine; vascular.
Conflict of interest statement
The authors have declared that no competing interest exits.
Figures
Similar articles
-
Facilitation of Insulin Effects by Ranolazine in Astrocytes in Primary Culture.Int J Mol Sci. 2022 Oct 9;23(19):11969. doi: 10.3390/ijms231911969. Int J Mol Sci. 2022. PMID: 36233271 Free PMC article.
-
Losartan improves aortic endothelium-dependent relaxation via proline-rich tyrosine kinase 2/Src/Akt pathway in type 2 diabetic Goto-Kakizaki rats.Am J Physiol Heart Circ Physiol. 2011 Dec;301(6):H2383-94. doi: 10.1152/ajpheart.00178.2011. Epub 2011 Sep 16. Am J Physiol Heart Circ Physiol. 2011. PMID: 21926342
-
Activation of the PDK-1/Akt/eNOS pathway involved in aortic endothelial function differs between hyperinsulinemic and insulin-deficient diabetic rats.Am J Physiol Heart Circ Physiol. 2009 Nov;297(5):H1767-75. doi: 10.1152/ajpheart.00536.2009. Epub 2009 Aug 28. Am J Physiol Heart Circ Physiol. 2009. PMID: 19717727
-
Akt/eNOS pathway activation in endothelium-dependent relaxation is preserved in aortas from female, but not from male, type 2 diabetic mice.Pharmacol Res. 2012 Jan;65(1):56-65. doi: 10.1016/j.phrs.2011.08.009. Epub 2011 Sep 14. Pharmacol Res. 2012. PMID: 21933713
-
Hypoglycaemia aggravates impaired endothelial-dependent vasodilation in diabetes by suppressing endothelial nitric oxide synthase activity and stimulating inducible nitric oxide synthase expression.Microvasc Res. 2023 Mar;146:104468. doi: 10.1016/j.mvr.2022.104468. Epub 2022 Dec 10. Microvasc Res. 2023. PMID: 36513147
References
-
- Wiecha J., Reineker K., Reitmayer M., Voisard R., Hannekum A., Mattfeldt T., Waltenberger J., Hombach V. Modulation of Ca2+-activated K+ channels in human vascular cells by insulin and basic fibroblast growth factor. Growth Horm. IGF Res. 1998;8:175–181. doi: 10.1016/S1096-6374(98)80108-1. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

