Pyk2/FAK Signaling Is Upregulated in Recurrent Glioblastoma Tumors in a C57BL/6/GL261 Glioma Implantation Model
- PMID: 37686276
- PMCID: PMC10487692
- DOI: 10.3390/ijms241713467
Pyk2/FAK Signaling Is Upregulated in Recurrent Glioblastoma Tumors in a C57BL/6/GL261 Glioma Implantation Model
Abstract
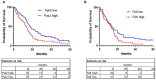
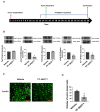
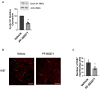
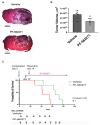
The majority of glioblastomas (GBMs) recur shortly after tumor resection and recurrent tumors differ significantly from newly diagnosed GBMs, phenotypically and genetically. In this study, using a Gl261-C57Bl/6 mouse glioma implantation model, we identified significant upregulation of proline-rich tyrosine kinase Pyk2 and focal adhesion kinase (FAK) phosphorylation levels-pPyk2 (579/580) and pFAK (925)-without significant modifications in total Pyk2 and FAK protein expression in tumors regrown after surgical resection, compared with primary implanted tumors. Previously, we demonstrated that Pyk2 and FAK are involved in the regulation of tumor cell invasion and proliferation and are associated with reduced overall survival. We hypothesized that the use of inhibitors of Pyk2/FAK in the postsurgical period may reduce the growth of recurrent tumors. Using Western blot analysis and confocal immunofluorescence approaches, we demonstrated upregulation of Cyclin D1 and the Ki67 proliferation index in tumors regrown after resection, compared with primary implanted tumors. Treatment with Pyk2/FAK inhibitor PF-562271, administered through oral gavage at 50 mg/kg daily for two weeks beginning 2 days before tumor resection, reversed Pyk2/FAK signaling upregulation in recurrent tumors, reduced tumor volume, and increased animal survival. In conclusion, the use of Pyk2/FAK inhibitors can contribute to a delay in GBM tumor regrowth after surgical resection.
Keywords: FAK; Pyk2; glioblastoma; recurrent tumor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
The PYK2 inhibitor PF-562271 enhances the effect of temozolomide on tumor growth in a C57Bl/6-Gl261 mouse glioma model.J Neurooncol. 2023 Feb;161(3):593-604. doi: 10.1007/s11060-023-04260-3. Epub 2023 Feb 15. J Neurooncol. 2023. PMID: 36790653 Free PMC article.
-
Microglia Activate Migration of Glioma Cells through a Pyk2 Intracellular Pathway.PLoS One. 2015 Jun 22;10(6):e0131059. doi: 10.1371/journal.pone.0131059. eCollection 2015. PLoS One. 2015. PMID: 26098895 Free PMC article.
-
Involvement of ROS-alpha v beta 3 integrin-FAK/Pyk2 in the inhibitory effect of melatonin on U251 glioma cell migration and invasion under hypoxia.J Transl Med. 2015 Mar 20;13:95. doi: 10.1186/s12967-015-0454-8. J Transl Med. 2015. PMID: 25889845 Free PMC article.
-
Focal Adhesion Kinases in Platelet Function and Thrombosis.Arterioscler Thromb Vasc Biol. 2019 May;39(5):857-868. doi: 10.1161/ATVBAHA.118.311787. Arterioscler Thromb Vasc Biol. 2019. PMID: 30894012 Review.
-
PYK2 and FAK in osteoclasts.Front Biosci. 2003 Sep 1;8:d1219-26. doi: 10.2741/1117. Front Biosci. 2003. PMID: 12957821 Review.
Cited by
-
The Potential Role of the Extracellular Matrix Glycoprotein Reelin in Glioblastoma Biology.Pharmaceuticals (Basel). 2024 Mar 21;17(3):401. doi: 10.3390/ph17030401. Pharmaceuticals (Basel). 2024. PMID: 38543187 Free PMC article.
References
-
- Ostrom Q.T., Gittleman H., Liao P., Rouse C., Chen Y., Dowling J., Wolinsky Y., Kruchko C., Barnholtz-Sloan J. CBTRUS Statistical Report: Primary brain and central nervous system tumors diagnosed in the United States in 2007–2011. Neuro-Oncology. 2014;16:iv1–iv63. doi: 10.1093/neuonc/nou223. - DOI - PMC - PubMed
-
- Dekker L.J.M., Kannegieter N.M., Haerkens F., Toth E., Kros J.M., Hov D.A.S., Fillebeen J., Verschuren L., Leenstra S., Ressa A., et al. Multiomics profiling of paired primary and recurrent glioblastoma patient tissues. Neuro-Oncol. Adv. 2020;2:vdaa083. doi: 10.1093/noajnl/vdaa083. - DOI - PMC - PubMed
-
- Sacko O., Benouaich-Amiel A., Brandicourt P., Niaré M., Charni S., Cavandoli C., Brauge D., Catalaa I., Brenner A., Moyal E.C.-J., et al. The Impact of Surgery on the Survival of Patients with Recurrent Glioblastoma. Asian J. Neurosurg. 2021;16:1–7. doi: 10.4103/ajns.AJNS_180_20. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

