Microtubule-Targeting Combined with HDAC Inhibition Is a Novel Therapeutic Strategy for Diffuse Intrinsic Pontine Gliomas
- PMID: 37683275
- PMCID: PMC10690044
- DOI: 10.1158/1535-7163.MCT-23-0179
Microtubule-Targeting Combined with HDAC Inhibition Is a Novel Therapeutic Strategy for Diffuse Intrinsic Pontine Gliomas
Abstract
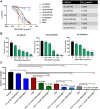
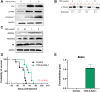
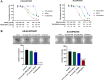
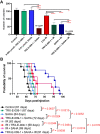
Diffuse intrinsic pontine gliomas (DIPG) are an incurable childhood brain cancer for which novel treatments are needed. DIPGs are characterized by a mutation in the H3 histone (H3K27M), resulting in loss of H3K27 methylation and global gene dysregulation. TRX-E-009-1 is a novel anticancer agent with preclinical activity demonstrated against a range of cancers. We examined the antitumor activity of TRX-E-009-1 against DIPG neurosphere cultures and observed tumor-specific activity with IC50s ranging from 20 to 100 nmol/L, whereas no activity was observed against normal human astrocyte cells. TRX-E-009-1 exerted its anti-proliferative effect through the induction of apoptotic pathways, with marked increases in cleaved caspase 3 and cleaved PARP levels, while also restoring histone H3K27me3 methylation. Co-administration of TRX-E-009-1 and the histone deacetylase (HDAC) inhibitor SAHA extended survival in DIPG orthotopic animal models. This antitumor effect was further enhanced with irradiation. Our findings indicate that TRX-E-009-1, combined with HDAC inhibition, represents a novel, potent therapy for children with DIPG.
©2023 The Authors; Published by the American Association for Cancer Research.
Figures

Similar articles
-
Dual targeting of the epigenome via FACT complex and histone deacetylase is a potent treatment strategy for DIPG.Cell Rep. 2021 Apr 13;35(2):108994. doi: 10.1016/j.celrep.2021.108994. Cell Rep. 2021. PMID: 33852836
-
Identification of Novel RAS Signaling Therapeutic Vulnerabilities in Diffuse Intrinsic Pontine Gliomas.Cancer Res. 2019 Aug 15;79(16):4026-4041. doi: 10.1158/0008-5472.CAN-18-3521. Epub 2019 Jun 14. Cancer Res. 2019. PMID: 31201162
-
Re-programing Chromatin with a Bifunctional LSD1/HDAC Inhibitor Induces Therapeutic Differentiation in DIPG.Cancer Cell. 2019 Nov 11;36(5):528-544.e10. doi: 10.1016/j.ccell.2019.09.005. Epub 2019 Oct 17. Cancer Cell. 2019. PMID: 31631026
-
Developing H3K27M mutant selective radiosensitization strategies in diffuse intrinsic pontine glioma.Neoplasia. 2023 Mar;37:100881. doi: 10.1016/j.neo.2023.100881. Epub 2023 Jan 30. Neoplasia. 2023. PMID: 36724689 Free PMC article. Review.
-
Epigenetic-Targeted Treatments for H3K27M-Mutant Midline Gliomas.Adv Exp Med Biol. 2021;1283:73-84. doi: 10.1007/978-981-15-8104-5_6. Adv Exp Med Biol. 2021. PMID: 33155139 Review.
Cited by
-
Single-cell landscape and spatial transcriptomic analysis reveals macrophage infiltration and glycolytic metabolism in kidney renal clear cell carcinoma.Aging (Albany NY). 2023 Oct 16;15(20):11298-11312. doi: 10.18632/aging.205128. Epub 2023 Oct 16. Aging (Albany NY). 2023. PMID: 37847178 Free PMC article.
References
-
- Donaldson SS, Laningham F, Fisher PG. Advances toward an understanding of brainstem gliomas. J Clin Oncol 2006;24:1266–72. - PubMed
-
- Jansen MHA, van Vuurden DG, Vandertop WP, Kaspers GJL. Diffuse intrinsic pontine gliomas: a systematic update on clinical trials and biology. Cancer Treat Rev 2012;38:27–35. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

