Safety and immunogenicity of a recombinant oligomeric gp145 subtype C Env protein (gp145 C.6980) HIV vaccine candidate in healthy, HIV-1-uninfected adult participants in the US
- PMID: 37679276
- PMCID: PMC11446254
- DOI: 10.1016/j.vaccine.2023.07.046
Safety and immunogenicity of a recombinant oligomeric gp145 subtype C Env protein (gp145 C.6980) HIV vaccine candidate in healthy, HIV-1-uninfected adult participants in the US
Abstract
Background: An approach to a preventive HIV vaccine is induction of effective broadly neutralizing antibodies (bnAbs) and effector binding antibodies (bAbs). Preclinical studies suggest that trimeric envelope (Env) proteins may elicit nAbs, which led to the development of the recombinant gp145 subtype C Env protein (gp145 C.6980) immunogen. HVTN 122 was a Phase 1 trial that evaluated the safety, tolerability, and immunogenicity of gp145 C.6980 in adults.
Methods: Healthy, HIV-1 seronegative adults received three intramuscular injections of gp145 C.6980 with aluminum hydroxide (alum) at months 0, 2, and 6 at either 300 mcg (high dose, n = 25) or 100 mcg (low dose, n = 15), or placebo/saline (placebo, n = 5). Participants were followed for 12 months.
Results: Forty-five participants were enrolled. High and low doses of the study protein were well-tolerated, with mild or moderate reactogenicity commonly reported. Only one adverse event (mild injection site pruritis) in one participant (low dose) was considered product-related; there were no dose-limiting toxicities. High and low dose recipients demonstrated robust bAb responses to vaccine-matched consensus gp140 Env and subtype-matched gp120 Env proteins two weeks post-last vaccination (response rates >90 %), while no responses were detected to a heterologous subtype-matched V1V2 antigen. No significant differences were seen between high and low dose groups. Participants in both experimental arms demonstrated nAb response rates of 76.5 % to a tier 1 virus (MW9635.26), but no responses to tier 2 isolates. Env-specific CD4 + T-cell responses were elicited in 36.4 % of vaccine recipients, without significant differences between groups; no participants demonstrated CD8 + T-cell responses.
Conclusions: Three doses of novel subtype C gp145 Env protein with alum were safe and well-tolerated. Participants demonstrated bAb, Env-specific CD4 + T-cell, and tier 1 nAb responses, but the regimen failed to induce tier 2 or heterologous nAb responses.
Clinical trials registration: NCT03382418.
Keywords: HIV vaccine; HIV-1; NIH; Phase 1 clinical trial; Subtype C gp145 Env protein; Trimeric envelope protein.
Copyright © 2023. Published by Elsevier Ltd.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

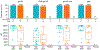

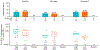

Similar articles
-
Comparable Antigenicity and Immunogenicity of Oligomeric Forms of a Novel, Acute HIV-1 Subtype C gp145 Envelope for Use in Preclinical and Clinical Vaccine Research.J Virol. 2015 Aug;89(15):7478-93. doi: 10.1128/JVI.00412-15. Epub 2015 May 13. J Virol. 2015. PMID: 25972551 Free PMC article.
-
Comparison of shortened mosaic HIV-1 vaccine schedules: a randomised, double-blind, placebo-controlled phase 1 trial (IPCAVD010/HPX1002) and a preclinical study in rhesus monkeys (NHP 17-22).Lancet HIV. 2020 Jun;7(6):e410-e421. doi: 10.1016/S2352-3018(20)30001-1. Epub 2020 Feb 17. Lancet HIV. 2020. PMID: 32078815 Free PMC article. Clinical Trial.
-
Safety and immune responses after a 12-month booster in healthy HIV-uninfected adults in HVTN 100 in South Africa: A randomized double-blind placebo-controlled trial of ALVAC-HIV (vCP2438) and bivalent subtype C gp120/MF59 vaccines.PLoS Med. 2020 Feb 24;17(2):e1003038. doi: 10.1371/journal.pmed.1003038. eCollection 2020 Feb. PLoS Med. 2020. PMID: 32092060 Free PMC article. Clinical Trial.
-
Safety and immunogenicity of a polyvalent DNA-protein HIV vaccine with matched Env immunogens delivered as a prime-boost regimen or coadministered in HIV-uninfected adults in the USA (HVTN 124): a phase 1, placebo-controlled, double-blind randomised controlled trial.Lancet HIV. 2024 May;11(5):e285-e299. doi: 10.1016/S2352-3018(24)00036-5. Lancet HIV. 2024. PMID: 38692824 Clinical Trial.
-
HIV Vaccination: A Roadmap among Advancements and Concerns.Int J Mol Sci. 2018 Apr 19;19(4):1241. doi: 10.3390/ijms19041241. Int J Mol Sci. 2018. PMID: 29671786 Free PMC article. Review.
References
-
- Hessell AJ, Hangartner L, Hunter M, et al. Fc receptor but not complement binding is important in antibody protection against HIV. Nature 2007; 449:101–4. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

