Regulation of the renin-angiotensin-aldosterone system by cyclic nucleotides and phosphodiesterases
- PMID: 37674612
- PMCID: PMC10478253
- DOI: 10.3389/fendo.2023.1239492
Regulation of the renin-angiotensin-aldosterone system by cyclic nucleotides and phosphodiesterases
Abstract
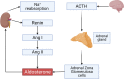
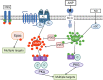
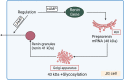
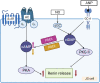
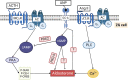
The renin-angiotensin-aldosterone system (RAAS) is one of the key players in the regulation of blood volume and blood pressure. Dysfunction of this system is connected with cardiovascular and renal diseases. Regulation of RAAS is under the control of multiple intracellular mechanisms. Cyclic nucleotides and phosphodiesterases are the major regulators of this system since they control expression and activity of renin and aldosterone. In this review, we summarize known mechanisms by which cyclic nucleotides and phosphodiesterases regulate renin gene expression, secretion of renin granules from juxtaglomerular cells and aldosterone production from zona glomerulosa cells of adrenal gland. We also discuss several open questions which deserve future attention.
Keywords: aldosterone; cAMP; cGMP; phosphodiesterase; renin.
Copyright © 2023 Gambaryan, Mohagaonkar and Nikolaev.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Regulation of the adrenal renin angiotensin system in cultured bovine zona glomerulosa cells: effect of catecholamines.Endocrinology. 1992 Apr;130(4):2129-34. doi: 10.1210/endo.130.4.1312445. Endocrinology. 1992. PMID: 1312445
-
Effects of atrial natriuretic factor on the renin-aldosterone system: in vivo and in vitro studies.J Steroid Biochem Mol Biol. 1993 Apr;45(1-3):173-8. doi: 10.1016/0960-0760(93)90138-m. J Steroid Biochem Mol Biol. 1993. PMID: 8386932 Review.
-
[Effect of short-term space flights on the activity of the renin-angiotensin-aldosterone system and on the blood concentration of cyclic nucleotides and prostaglandins].Kosm Biol Aviakosm Med. 1986 Sep-Oct;20(5):27-30. Kosm Biol Aviakosm Med. 1986. PMID: 3784519 Russian.
-
New approaches to blockade of the renin-angiotensin-aldosterone system: overview of regulation of the renin-angiotensin-aldosterone system.J Pharmacol Sci. 2010;113(4):289-91. doi: 10.1254/jphs.10r03fm. Epub 2010 Jul 27. J Pharmacol Sci. 2010. PMID: 20675960 Review.
-
Angiotensin II and aldosterone regulation.Regul Pept. 1999 Mar 17;80(1-2):27-32. doi: 10.1016/s0167-0115(99)00004-x. Regul Pept. 1999. PMID: 10235631 Review.
Cited by
-
Oral Supplementation with Betaine Powder Ameliorated High Blood Pressure in Spontaneously Hypertensive Rats.Metabolites. 2024 Jul 18;14(7):390. doi: 10.3390/metabo14070390. Metabolites. 2024. PMID: 39057713 Free PMC article.
-
Kidney Renin Release under Hypoxia and Its Potential Link with Nitric Oxide: A Narrative Review.Biomedicines. 2023 Nov 6;11(11):2984. doi: 10.3390/biomedicines11112984. Biomedicines. 2023. PMID: 38001984 Free PMC article. Review.
-
Molecular and Genetics Perspectives on Primary Adrenocortical Hyperfunction Disorders.Int J Mol Sci. 2024 Oct 22;25(21):11341. doi: 10.3390/ijms252111341. Int J Mol Sci. 2024. PMID: 39518893 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

