Dapagliflozin impedes endothelial cell senescence by activating the SIRT1 signaling pathway in type 2 diabetes
- PMID: 37664712
- PMCID: PMC10469571
- DOI: 10.1016/j.heliyon.2023.e19152
Dapagliflozin impedes endothelial cell senescence by activating the SIRT1 signaling pathway in type 2 diabetes
Abstract
Background: Sodium-glucose cotransporter 2 inhibitors (SGLT2i) clinically reduce atherosclerosis and lower blood pressure. However, their impact on endothelial dysfunction in type 2 diabetes (T2D) remains unclear. In this study, we investigated the protective effect and underlying mechanism of the SGLT2 inhibitor dapagliflozin in diabetes.
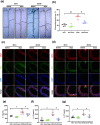
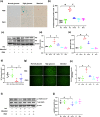
Methods: Vascular reactivity was measured to assess the vasoprotective effect of dapagliflozin in a mouse model of high glucose (HG)-induced T2D. Pulse wave velocity was measured to quantify arterial stiffness. Protein expression was assessed by western blotting and immunofluorescence, oxidative stress was evaluated using dihydroethidium, nitric oxide was evaluated using the Griess reaction, and cellular senescence was assessed based on senescence-associated beta-galactosidase (SA-β-gal) activity and the expression of senescence markers. Furthermore, the endothelial nitric oxide synthase (eNOS) acetylation status was determined and eNOS interactions with SIRT1 were evaluated by coimmunoprecipitation assays.
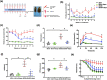
Results: Dapagliflozin protected against impaired endothelium-dependent vasorelaxation and improved arterial stiffness in the mouse model of T2D; mouse aortas had significantly reduced levels of senescence activity and senescence-associated inflammatory factors. HG-induced increases in senescence activity, protein marker levels, and oxidative stress in vitro were all ameliorated by dapagliflozin. The decreases in eNOS phosphorylation and nitric oxide (NO) production in senescent endothelial cells were restored by dapagliflozin. SIRT1 expression was reduced in HG-induced senescent endothelial cells, and dapagliflozin restored SIRT1 expression. SIRT1 inhibition diminished the antisenescence effects of dapagliflozin. Coimmunoprecipitation showed that SIRT1 was physically associated with eNOS, suggesting that the effects of dapagliflozin are dependent on SIRT1 activation.
Conclusion: These findings indicate that dapagliflozin protects against endothelial cell senescence by regulating SIRT1 signaling in diabetic mice.
Keywords: Dapagliflozin; Diabetes; Endothelial cells; SGLT2 inhibitor; SIRT1; Senescence.
© 2023 The Authors.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Dapagliflozin prevents oxidative stress-induced endothelial dysfunction via sirtuin 1 activation.Biomed Pharmacother. 2023 Sep;165:115213. doi: 10.1016/j.biopha.2023.115213. Epub 2023 Jul 28. Biomed Pharmacother. 2023. PMID: 37517289
-
Anthocyanins attenuate endothelial dysfunction through regulation of uncoupling of nitric oxide synthase in aged rats.Aging Cell. 2020 Dec;19(12):e13279. doi: 10.1111/acel.13279. Epub 2020 Dec 3. Aging Cell. 2020. PMID: 33274583 Free PMC article.
-
[Tanshinone IIA attenuates hydrogen peroxide-induced senescence of human umbilical vein endothelial cells through activating SIRT1/eNOS pathway].Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2019 Sep;35(9):806-811. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2019. PMID: 31750822 Chinese.
-
Metformin modulates hyperglycaemia-induced endothelial senescence and apoptosis through SIRT1.Br J Pharmacol. 2014 Jan;171(2):523-35. doi: 10.1111/bph.12496. Br J Pharmacol. 2014. PMID: 24372553 Free PMC article.
-
Protective Role of Endogenous Kallistatin in Vascular Injury and Senescence by Inhibiting Oxidative Stress and Inflammation.Oxid Med Cell Longev. 2018 Dec 2;2018:4138560. doi: 10.1155/2018/4138560. eCollection 2018. Oxid Med Cell Longev. 2018. PMID: 30622668 Free PMC article. Review.
Cited by
-
Antihypertensive Effects of SGLT2-Inhibitors: Considerations for Clinical Practice.Curr Vasc Pharmacol. 2024;22(4):231-233. doi: 10.2174/0115701611274645231208102130. Curr Vasc Pharmacol. 2024. PMID: 39300714 No abstract available.
-
Tofogliflozin Delays Portal Hypertension and Hepatic Fibrosis by Inhibiting Sinusoidal Capillarization in Cirrhotic Rats.Cells. 2024 Mar 19;13(6):538. doi: 10.3390/cells13060538. Cells. 2024. PMID: 38534382 Free PMC article.
-
Aging in Ocular Blood Vessels: Molecular Insights and the Role of Oxidative Stress.Biomedicines. 2024 Apr 8;12(4):817. doi: 10.3390/biomedicines12040817. Biomedicines. 2024. PMID: 38672172 Free PMC article. Review.
-
The Multifaceted Role of Endothelial Sirt1 in Vascular Aging: An Update.Cells. 2024 Sep 1;13(17):1469. doi: 10.3390/cells13171469. Cells. 2024. PMID: 39273039 Free PMC article. Review.
-
SGLT2i improves kidney senescence by down-regulating the expression of LTBP2 in SAMP8 mice.J Cell Mol Med. 2024 Mar;28(6):e18176. doi: 10.1111/jcmm.18176. J Cell Mol Med. 2024. PMID: 38454800 Free PMC article.
References
-
- Menke A., Casagrande S., Cowie C.C. Prevalence of diabetes in adolescents aged 12 to 19 Years in the United States, 2005-2014. JAMA. 2016;316(3):344–345. - PubMed
-
- Cho N.H., Shaw J.E., Karuranga S., et al. IDF Diabetes Atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018;138:271–281. - PubMed
-
- Virani S.S., Alonso A., Benjamin E.J., et al. Heart disease and stroke statistics-2020 update: a report from the American heart association. Circulation. 2020;141(9):e139–e596. - PubMed
LinkOut - more resources
Full Text Sources

