Nicotinamide enhances myelin production after demyelination through reduction of astrogliosis and microgliosis
- PMID: 37663127
- PMCID: PMC10469866
- DOI: 10.3389/fncel.2023.1201317
Nicotinamide enhances myelin production after demyelination through reduction of astrogliosis and microgliosis
Abstract
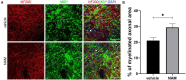
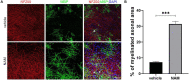
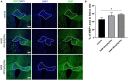
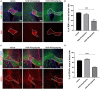
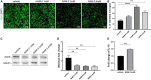
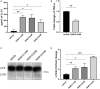
Caloric restriction is the chronic reduction of total caloric intake without malnutrition and has attracted a lot of attention as, among multiple other effects, it attenuates demyelination and stimulates remyelination. In this study we have evaluated the effect of nicotinamide (NAM), a well-known caloric restriction mimetic, on myelin production upon demyelinating conditions. NAM is the derivative of nicotinic acid (vitamin B3) and a precursor of nicotinamide adenine dinucleotide (NAD+), a ubiquitous metabolic cofactor. Here, we use cortical slices ex vivo subjected to demyelination or cultured upon normal conditions, a lysolecithin (LPC)-induced focal demyelination mouse model as well as primary glial cultures. Our data show that NAM enhances both myelination and remyelination ex vivo, while it also induces myelin production after LPC-induced focal demyelination ex vivo and in vivo. The increased myelin production is accompanied by reduction in both astrogliosis and microgliosis in vivo. There is no direct effect of NAM on the oligodendrocyte lineage, as no differences are observed in oligodendrocyte precursor cell proliferation or differentiation or in the number of mature oligodendrocytes. On the other hand, NAM affects both microglia and astrocytes as it decreases the population of M1-activated microglia, while reducing the pro-inflammatory phenotype of astrocytes as assayed by the reduction of TNF-α. Overall, we show that the increased myelin production that follows NAM treatment in vivo is accompanied by a decrease in both astrocyte and microglia accumulation at the lesion site. Our data indicate that NAM influences astrocytes and microglia directly, in favor of the remyelination process by promoting a less inflammatory environment.
Keywords: astrocytes; caloric restriction; microglia; myelin; nicotinamide (NAM); remyelination.
Copyright © 2023 Kaplanis, Kaffe, Ktena, Lygeraki, Kolliniati, Savvaki and Karagogeos.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
The Roles of Caloric Restriction Mimetics in Central Nervous System Demyelination and Remyelination.Curr Issues Mol Biol. 2023 Nov 27;45(12):9526-9548. doi: 10.3390/cimb45120596. Curr Issues Mol Biol. 2023. PMID: 38132442 Free PMC article. Review.
-
Critical Role of Astrocyte NAD+ Glycohydrolase in Myelin Injury and Regeneration.J Neurosci. 2021 Oct 13;41(41):8644-8667. doi: 10.1523/JNEUROSCI.2264-20.2021. Epub 2021 Sep 7. J Neurosci. 2021. PMID: 34493542 Free PMC article.
-
Astrocytes regulate myelin clearance through recruitment of microglia during cuprizone-induced demyelination.Brain. 2013 Jan;136(Pt 1):147-67. doi: 10.1093/brain/aws262. Epub 2012 Dec 24. Brain. 2013. PMID: 23266461
-
S100B Impairs Oligodendrogenesis and Myelin Repair Following Demyelination Through RAGE Engagement.Front Cell Neurosci. 2020 Sep 4;14:279. doi: 10.3389/fncel.2020.00279. eCollection 2020. Front Cell Neurosci. 2020. PMID: 33100970 Free PMC article.
-
The roles of microglia and astrocytes in phagocytosis and myelination: Insights from the cuprizone model of multiple sclerosis.Glia. 2022 Jul;70(7):1215-1250. doi: 10.1002/glia.24148. Epub 2022 Feb 2. Glia. 2022. PMID: 35107839 Free PMC article. Review.
Cited by
-
The Roles of Caloric Restriction Mimetics in Central Nervous System Demyelination and Remyelination.Curr Issues Mol Biol. 2023 Nov 27;45(12):9526-9548. doi: 10.3390/cimb45120596. Curr Issues Mol Biol. 2023. PMID: 38132442 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources

