Human cytomegalovirus infection enhances 5‑lipoxygenase and cycloxygenase‑2 expression in colorectal cancer
- PMID: 37654195
- PMCID: PMC10546380
- DOI: 10.3892/ijo.2023.5564
Human cytomegalovirus infection enhances 5‑lipoxygenase and cycloxygenase‑2 expression in colorectal cancer
Abstract
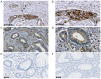
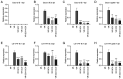
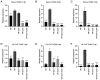
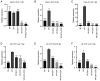
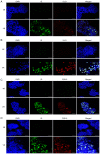
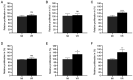
Colorectal cancer (CRC) is one of the most common and fatal types of cancer. Inflammation promotes CRC development, however, the underlying etiological factors are unknown. Human cytomegalovirus (HCMV), a virus that induces inflammation and other cancer hallmarks, has been detected in several types of malignancy, including CRC. The present study investigated whether HCMV infection was associated with expression of the pro‑inflammatory enzymes 5‑lipoxygenase (5‑LO) and cyclooxygenase‑2 (COX‑2) and other molecular, genetic and clinicopathological CRC features. The present study assessed 146 individual paraffin‑embedded CRC tissue microarray (TMA) cores already characterized for TP53 and KRAS mutations, microsatellite instability (MSI) status, Ki‑67 index and EGFR by immunohistochemistry (IHC). The cores were further analyzed by IHC for the expression of two HCMV proteins (Immediate Early, IE and pp65) and the inflammatory markers 5‑LO and COX‑2. The CRC cell lines Caco‑2 and LS‑174T were infected with HCMV strain VR1814, treated with antiviral drug ganciclovir (GCV) and/or anti‑inflammatory drug celecoxib (CCX) and analyzed by reverse transcription‑quantitative PCR and immunofluorescence for 5‑LO, COX‑2, IE and pp65 transcripts and proteins. HCMV IE and pp65 proteins were detected in ~90% of the CRC cases tested; this was correlated with COX‑2, 5‑LO and KI‑67 expression, but not with EGFR immunostaining, TP53 and KRAS mutations or MSI status. In vitro, HCMV infection upregulated 5‑LO and COX‑2 transcript and proteins in both Caco‑2 and LS‑174T cells and enhanced cell proliferation as determined by MTT assay. Treatment with GCV and CCX significantly decreased the transcript levels of COX‑2, 5‑LO, HCMV IE and pp65 in infected cells. HCMV was widely expressed in CRC and may promote inflammation and serve as a potential new target for CRC therapy.
Keywords: celecoxib; colorectal cancer; ganciclovir; human cytomegalovirus; inflammation.
Conflict of interest statement
CSN holds a patent on diagnostics and treatment of a CMV variant strain found in cancer (patent no. US9701943B2). The other authors declare that they have no competing interests.
Figures

Similar articles
-
Evidence of human cytomegalovirus infection and expression of 5-lipoxygenase in borderline ovarian tumors.J Med Virol. 2021 Jun;93(6):4023-4027. doi: 10.1002/jmv.26664. Epub 2020 Nov 22. J Med Virol. 2021. PMID: 33174621
-
Human cytomegalovirus infection enhances cell proliferation, migration and upregulation of EMT markers in colorectal cancer-derived stem cell-like cells.Int J Oncol. 2017 Nov;51(5):1415-1426. doi: 10.3892/ijo.2017.4135. Epub 2017 Sep 25. Int J Oncol. 2017. PMID: 29048611 Free PMC article.
-
Detection of Human cytomegalovirus UL55 Gene and IE/E Protein Expression in Colorectal Cancer Patients in Egypt.BMC Cancer. 2023 Aug 3;23(1):723. doi: 10.1186/s12885-023-11200-x. BMC Cancer. 2023. PMID: 37537552 Free PMC article.
-
Human cytomegalovirus infection is correlated with enhanced cyclooxygenase-2 and 5-lipoxygenase protein expression in breast cancer.J Cancer Res Clin Oncol. 2019 Aug;145(8):2083-2095. doi: 10.1007/s00432-019-02946-8. Epub 2019 Jun 15. J Cancer Res Clin Oncol. 2019. PMID: 31203442 Free PMC article.
-
Ganciclovir resistance as a result of oral ganciclovir in a heart transplant recipient with multiple human cytomegalovirus strains in blood.Transplantation. 1998 Aug 15;66(3):324-9. doi: 10.1097/00007890-199808150-00008. Transplantation. 1998. PMID: 9721800
Cited by
-
Exploring Gut Microbiome Composition and Circulating Microbial DNA Fragments in Patients with Stage II/III Colorectal Cancer: A Comprehensive Analysis.Cancers (Basel). 2024 May 18;16(10):1923. doi: 10.3390/cancers16101923. Cancers (Basel). 2024. PMID: 38792001 Free PMC article.
References
-
- Jeon J, Du M, Schoen RE, Hoffmeister M, Newcomb PA, Berndt SI, Caan B, Campbell PT, Chan AT, Chang-Claude J, et al. Determining risk of colorectal cancer and starting age of screening based on lifestyle, environmental, and genetic factors. Gastroenterology. 2018;154:2152–2164.e2119. doi: 10.1053/j.gastro.2018.02.021. - DOI - PMC - PubMed
-
- Dolin TG, Christensen IJ, Johansen AZ, Nielsen HJ, Jakobsen HL, Klein MF, Lund CM, Bojesen SE, Nielsen DL, Jensen BV, Johansen JS. Pre- and perioperative inflammatory biomarkers in older patients resected for localized colorectal cancer: Associations with complications and prognosis. Cancers (Basel) 2022;14:161. doi: 10.3390/cancers14010161. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
