Screening of heat-killed lactic acid bacteria based on inhibitory activity against oral bacteria and effects of oral administration of heat-killed Ligilactobacillus salivarius CP3365 on periodontal health in healthy participants: a double-blinded, randomized, placebo-controlled trial
- PMID: 37649969
- PMCID: PMC10464545
- DOI: 10.1080/20002297.2023.2250649
Screening of heat-killed lactic acid bacteria based on inhibitory activity against oral bacteria and effects of oral administration of heat-killed Ligilactobacillus salivarius CP3365 on periodontal health in healthy participants: a double-blinded, randomized, placebo-controlled trial
Abstract
Objectives: The aims of this study were to select heat-killed lactic acid bacteria (HKL) with antibiotic activity and investigate the efficacy of this bacteria in maintaining periodontal parameters in healthy participants.
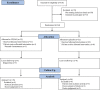
Materials and methods: An in vitro evaluation was conducted to assess the inhibitory efficacy of lactic acid bacteria against Porphyromonas gingivalis and Fusobacterium nucleatum subsp. nucleatum. The effects of HKL administration on various parameters (plaque control record, bleeding on probing, and probing pocket depth) were assessed in a randomized, placebo-controlled trial. Participants in the test and placebo groups (n = 32) consumed oral tablets containing placebo or HKL daily for 8 weeks. Oral bacteria in supra-plaque and saliva were identified using 16S rRNA gene community profiling analysis.
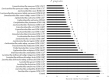
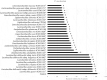
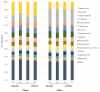
Results: Heat-killed Ligilactobacillus salivarius CP3365 significantly (p < 0.05) decreased the viability of oral bacteria and was selected for clinical trials. Administration of HKL CP3365 significantly (p < 0.05) inhibited increases in each parameter. No changes in the relative abundance of P. gingivalis or F. nucleatum subsp. nucleatum were detected by HKL CP3365, but the relative abundance of oral bacteria (genera Porphyromonas, Fusobacterium, and Haemophilus) was significantly (p < 0.05) decreased.
Conclusion: HKL CP3365 effectively inhibited oral bacteria growth and was useful for maintaining periodontal health.
Clinical trial registration: [https://www.umin.ac.jp/ctr/index.htm], identifier [UMIN000045656].
Keywords: Bleeding on probing (BOP); Ligilactobacillus salivarius CP3365; dental plaque; heat-killed lactic acid bacteria; probing pocket depth (PPD).
© 2023 The Author(s). Published by Informa UK Limited, trading as Taylor & Francis Group.
Conflict of interest statement
S.S., Y. S., M.Y., and T.H. are employees of Asahi Quality and Innovations Ltd., which is affiliated with Asahi Group Holdings Ltd., and they prepared CP3365 and the placebo. T.S receives research consulting fees from Kyowa Trial Co., Ltd.
Figures


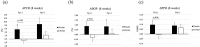
Similar articles
-
Antibacterial activity of viable and heat-killed probiotic strains against oral pathogens.Lett Appl Microbiol. 2020 Apr;70(4):310-317. doi: 10.1111/lam.13275. Epub 2020 Feb 11. Lett Appl Microbiol. 2020. PMID: 31955445
-
Effects of oil drops containing Lactobacillus salivarius WB21 on periodontal health and oral microbiota producing volatile sulfur compounds.J Breath Res. 2012 Mar;6(1):017106. doi: 10.1088/1752-7155/6/1/017106. Epub 2012 Feb 27. J Breath Res. 2012. PMID: 22368259 Clinical Trial.
-
Clinical and microbiological effects of initial periodontal therapy in conjunction with amoxicillin and clavulanic acid in patients with adult periodontitis. A randomised double-blind, placebo-controlled study.J Clin Periodontol. 1999 Jul;26(7):461-8. doi: 10.1034/j.1600-051x.1999.260708.x. J Clin Periodontol. 1999. PMID: 10412851 Clinical Trial.
-
Can metagenomics unravel the impact of oral bacteriome in human diseases?Biotechnol Genet Eng Rev. 2023 Apr;39(1):85-117. doi: 10.1080/02648725.2022.2102877. Epub 2022 Jul 21. Biotechnol Genet Eng Rev. 2023. PMID: 35861776 Review.
-
Full-mouth treatment modalities (within 24 hours) for chronic periodontitis in adults.Cochrane Database Syst Rev. 2015 Apr 17;2015(4):CD004622. doi: 10.1002/14651858.CD004622.pub3. Cochrane Database Syst Rev. 2015. Update in: Cochrane Database Syst Rev. 2022 Jun 28;6:CD004622. doi: 10.1002/14651858.CD004622.pub4. PMID: 25884249 Free PMC article. Updated. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
