The rapid emergence of antifungal-resistant human-pathogenic fungi
- PMID: 37648790
- PMCID: PMC10859884
- DOI: 10.1038/s41579-023-00960-9
The rapid emergence of antifungal-resistant human-pathogenic fungi
Abstract
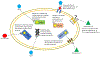
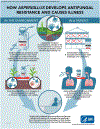
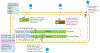
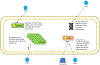
During recent decades, the emergence of pathogenic fungi has posed an increasing public health threat, particularly given the limited number of antifungal drugs available to treat invasive infections. In this Review, we discuss the global emergence and spread of three emerging antifungal-resistant fungi: Candida auris, driven by global health-care transmission and possibly facilitated by climate change; azole-resistant Aspergillus fumigatus, driven by the selection facilitated by azole fungicide use in agricultural and other settings; and Trichophyton indotineae, driven by the under-regulated use of over-the-counter high-potency corticosteroid-containing antifungal creams. The diversity of the fungi themselves and the drivers of their emergence make it clear that we cannot predict what might emerge next. Therefore, vigilance is critical to monitoring fungal emergence, as well as the rise in overall antifungal resistance.
© 2023. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
Figures


Similar articles
-
Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review.
-
Antifungal resistance in dermatophytes - review of the epidemiology, diagnostic challenges and treatment strategies for managing Trichophyton indotineae infections.Expert Rev Anti Infect Ther. 2024 Sep;22(9):739-751. doi: 10.1080/14787210.2024.2390629. Epub 2024 Aug 18. Expert Rev Anti Infect Ther. 2024. PMID: 39114868 Review.
-
Valley fever under a changing climate in the United States.Environ Int. 2024 Nov;193:109066. doi: 10.1016/j.envint.2024.109066. Epub 2024 Oct 11. Environ Int. 2024. PMID: 39432997 Review.
-
Qualitative evidence synthesis informing our understanding of people's perceptions and experiences of targeted digital communication.Cochrane Database Syst Rev. 2019 Oct 23;10(10):ED000141. doi: 10.1002/14651858.ED000141. Cochrane Database Syst Rev. 2019. PMID: 31643081 Free PMC article.
-
The Two-Component Response Regulator Ssk1 and the Mitogen-Activated Protein Kinase Hog1 Control Antifungal Drug Resistance and Cell Wall Architecture of Candida auris.mSphere. 2020 Oct 14;5(5):e00973-20. doi: 10.1128/mSphere.00973-20. mSphere. 2020. PMID: 33055262 Free PMC article.
Cited by
-
Application of Raman spectroscopy and machine learning for Candida auris identification and characterization.Appl Environ Microbiol. 2024 Nov 20;90(11):e0102524. doi: 10.1128/aem.01025-24. Epub 2024 Oct 29. Appl Environ Microbiol. 2024. PMID: 39470219
-
The influence of urban environmental effects on the orchard soil microbial community structure and function: a case study in Zhejiang, China.Front Microbiol. 2024 Sep 9;15:1403443. doi: 10.3389/fmicb.2024.1403443. eCollection 2024. Front Microbiol. 2024. PMID: 39314879 Free PMC article.
-
Mycologists and Virologists Align: Proposing Botrytis cinerea for Global Mycovirus Studies.Viruses. 2024 Sep 18;16(9):1483. doi: 10.3390/v16091483. Viruses. 2024. PMID: 39339959 Free PMC article. Review.
-
Deep learning-driven imaging of cell division and cell growth across an entire eukaryotic life cycle.bioRxiv [Preprint]. 2024 Apr 27:2024.04.25.591211. doi: 10.1101/2024.04.25.591211. bioRxiv. 2024. PMID: 38712227 Free PMC article. Preprint.
-
The pathobiology of human fungal infections.Nat Rev Microbiol. 2024 Nov;22(11):687-704. doi: 10.1038/s41579-024-01062-w. Epub 2024 Jun 25. Nat Rev Microbiol. 2024. PMID: 38918447 Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

