Modeling human brain rhabdoid tumor by inactivating tumor suppressor genes in induced pluripotent stem cells
- PMID: 37637078
- PMCID: PMC10448240
- DOI: 10.1016/j.bioactmat.2023.08.009
Modeling human brain rhabdoid tumor by inactivating tumor suppressor genes in induced pluripotent stem cells
Abstract
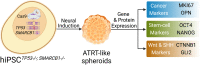
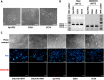
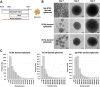
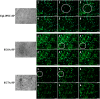
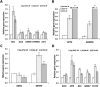
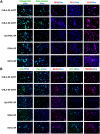
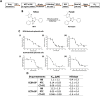
Atypical teratoid/rhabdoid tumor (ATRT) is a rare childhood malignancy that originates in the central nervous system. Over ninety-five percent of ATRT patients have biallelic inactivation of the tumor suppressor gene SMARCB1. ATRT has no standard treatment, and a major limiting factor in therapeutic development is the lack of reliable ATRT models. We employed CRISPR/Cas9 gene-editing technology to knock out SMARCB1 and TP53 genes in human episomal induced pluripotent stem cells (Epi-iPSCs), followed by brief neural induction, to generate an ATRT-like model. The dual knockout Epi-iPSCs retained their stemness with the capacity to differentiate into three germ layers. High expression of OCT4 and NANOG in neurally induced knockout spheroids was comparable to that in two ATRT cell lines. Beta-catenin protein expression was higher in SMARCB1-deficient cells and spheroids than in normal Epi-iPSC-derived spheroids. Nucleophosmin, Osteopontin, and Ki-67 proteins were also expressed by the SMARCB1-deficient spheroids. In summary, the tumor model resembled embryonal features of ATRT and expressed ATRT biomarkers at mRNA and protein levels. Ribociclib, PTC-209, and the combination of clofilium tosylate and pazopanib decreased the viability of the ATRT-like cells. This disease modeling scheme may enable the establishment of individualized tumor models with patient-specific mutations and facilitate high-throughput drug testing.
Keywords: Atypical teratoid/rhabdoid tumor; Brain tumor modeling; CRISPR/Cas9 gene editing; Human induced pluripotent stem cells; SMARCB1; Tumor suppressor genes.
© 2023 The Authors.
Conflict of interest statement
None.
Figures




Similar articles
-
Human Malignant Rhabdoid Tumor Antigens as Biomarkers and Potential Therapeutic Targets.Cancers (Basel). 2022 Jul 28;14(15):3685. doi: 10.3390/cancers14153685. Cancers (Basel). 2022. PMID: 35954348 Free PMC article.
-
Functional relevance of genes predicted to be affected by epigenetic alterations in atypical teratoid/rhabdoid tumors.J Neurooncol. 2019 Jan;141(1):43-55. doi: 10.1007/s11060-018-03018-6. Epub 2018 Nov 16. J Neurooncol. 2019. PMID: 30446899
-
SMARCB1 Gene Therapy Using a Novel Tumor-Targeted Nanomedicine Enhances Anti-Cancer Efficacy in a Mouse Model of Atypical Teratoid Rhabdoid Tumors.Int J Nanomedicine. 2024 Jun 14;19:5973-5993. doi: 10.2147/IJN.S458323. eCollection 2024. Int J Nanomedicine. 2024. PMID: 38895149 Free PMC article.
-
Atypical Teratoid/Rhabdoid Sellar Tumor in an Adult with a Familial History of a Germline SMARCB1 Mutation: Case Report and Review of the Literature.World Neurosurg. 2019 Jul;127:336-345. doi: 10.1016/j.wneu.2019.04.083. Epub 2019 Apr 17. World Neurosurg. 2019. PMID: 31004861 Review.
-
Atypical teratoid rhabdoid tumor: molecular insights and translation to novel therapeutics.J Neurooncol. 2020 Oct;150(1):47-56. doi: 10.1007/s11060-020-03639-w. Epub 2020 Oct 6. J Neurooncol. 2020. PMID: 33021733 Free PMC article. Review.
References
-
- Ramli R.A., et al. Identification of the cellular origin and" stemness" phenotype of Malignant Rhabdoid Tumors (MRT) may represent a new therapeutic approach in paediatric oncology. Cancer Res. 2017;77 4875-4875.
-
- Terada Y., et al. Human pluripotent stem cell-derived tumor model uncovers the embryonic stem cell signature as a key driver in atypical teratoid/rhabdoid tumor. Cell Rep. 2019;26:2608–2621. e2606. - PubMed
-
- Rorke L.B., Packer R.J., Biegel J.A. Central nervous system atypical teratoid/rhabdoid tumors of infancy and childhood: definition of an entity. J. Neurosurg. 1996;85:56–65. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

