Lipid-hybrid cell-derived biomimetic functional materials: A state-of-the-art multifunctional weapon against tumors
- PMID: 37636983
- PMCID: PMC10448342
- DOI: 10.1016/j.mtbio.2023.100751
Lipid-hybrid cell-derived biomimetic functional materials: A state-of-the-art multifunctional weapon against tumors
Abstract
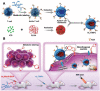
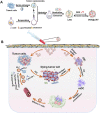
Tumors are among the leading causes of death worldwide. Cell-derived biomimetic functional materials have shown great promise in the treatment of tumors. These materials are derived from cell membranes, extracellular vesicles and bacterial outer membrane vesicles and may evade immune recognition, improve drug targeting and activate antitumor immunity. However, their use is limited owing to their low drug-loading capacity and complex preparation methods. Liposomes are artificial bionic membranes that have high drug-loading capacity and can be prepared and modified easily. Although they can overcome the disadvantages of cell-derived biomimetic functional materials, they lack natural active targeting ability. Lipids can be hybridized with cell membranes, extracellular vesicles or bacterial outer membrane vesicles to form lipid-hybrid cell-derived biomimetic functional materials. These materials negate the disadvantages of both liposomes and cell-derived components and represent a promising delivery platform in the treatment of tumors. This review focuses on the design strategies, applications and mechanisms of action of lipid-hybrid cell-derived biomimetic functional materials and summarizes the prospects of their further development and the challenges associated with it.
Keywords: Bacterial outer membrane vesicles; Cell membranes; Extracellular vesicles; Hybrid; Liposomes; Tumors therapy.
© 2023 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



Similar articles
-
Hybrid cell membrane-coated nanoparticles: A multifunctional biomimetic platform for cancer diagnosis and therapy.Acta Biomater. 2020 Aug;112:1-13. doi: 10.1016/j.actbio.2020.05.028. Epub 2020 May 26. Acta Biomater. 2020. PMID: 32470527 Review.
-
Cell-derived biomimetic nanocarriers for targeted cancer therapy: cell membranes and extracellular vesicles.Drug Deliv. 2021 Dec;28(1):1237-1255. doi: 10.1080/10717544.2021.1938757. Drug Deliv. 2021. PMID: 34142930 Free PMC article. Review.
-
Biomembrane-based nanostructures for cancer targeting and therapy: From synthetic liposomes to natural biomembranes and membrane-vesicles.Adv Drug Deliv Rev. 2021 Nov;178:113974. doi: 10.1016/j.addr.2021.113974. Epub 2021 Sep 13. Adv Drug Deliv Rev. 2021. PMID: 34530015 Review.
-
Phytochemicals and Cancer Treatment: Cell-Derived and Biomimetic Vesicles as Promising Carriers.Pharmaceutics. 2023 May 9;15(5):1445. doi: 10.3390/pharmaceutics15051445. Pharmaceutics. 2023. PMID: 37242687 Free PMC article. Review.
-
Construction of Biomimetic-Responsive Nanocarriers and their Applications in Tumor Targeting.Anticancer Agents Med Chem. 2022;22(12):2255-2273. doi: 10.2174/1871520622666220106105315. Anticancer Agents Med Chem. 2022. PMID: 34994336
Cited by
-
Targeted blood-brain barrier penetration and precise imaging of infiltrative glioblastoma margins using hybrid cell membrane-coated ICG liposomes.J Nanobiotechnology. 2024 Oct 5;22(1):603. doi: 10.1186/s12951-024-02870-1. J Nanobiotechnology. 2024. PMID: 39367395 Free PMC article.
-
Engineered Cell Membrane-Camouflaged Nanomaterials for Biomedical Applications.Nanomaterials (Basel). 2024 Feb 23;14(5):413. doi: 10.3390/nano14050413. Nanomaterials (Basel). 2024. PMID: 38470744 Free PMC article. Review.
-
Biomimetic Ghost Nanomedicine-Based Optotheranostics for Cancer.Nano Lett. 2024 Jul 10;24(27):8217-8231. doi: 10.1021/acs.nanolett.4c01534. Epub 2024 Jun 7. Nano Lett. 2024. PMID: 38848540 Free PMC article. Review.
-
Stem cell technology for antitumor drug loading and delivery in oncology.Oncol Res. 2024 Feb 6;32(3):433-437. doi: 10.32604/or.2023.046497. eCollection 2024. Oncol Res. 2024. PMID: 38361752 Free PMC article. Review.
-
Research progress of cell membrane biomimetic nanoparticles for circulating tumor cells.Front Oncol. 2024 Apr 30;14:1389775. doi: 10.3389/fonc.2024.1389775. eCollection 2024. Front Oncol. 2024. PMID: 38746681 Free PMC article. Review.
References
-
- Siegel R.L., Miller K.D., Wagle N.S., Jemal A. Cancer statistics, 2023. CA A Cancer J. Clin. 2023;73(1):17–48. - PubMed
-
- Stribbling S.M., Ryan A.J. The cell-line-derived subcutaneous tumor model in preclinical cancer research. Nat. Protoc. 2022;17(9):2108–2128. - PubMed
-
- Ryu J.H., Yoon H.Y., Sun I.C., Kwon I.C., Kim K. Tumor-targeting glycol chitosan nanoparticles for cancer heterogeneity. Adv. Mater. 2020;32(51) - PubMed
-
- AlQahtani S.A., Harisa G.I., Badran M.M., AlGhamdi K.M., Kumar A., Salem-Bekhit M.M., Ahmad S.F., Alanazi F.K. Nano-erythrocyte membrane-chaperoned 5-fluorouracil liposomes as biomimetic delivery platforms to target hepatocellular carcinoma cell lines. Artif. Cell Nanomed. Biotechnol. 2019;47(1):989–996. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

