Nox1-based NADPH oxidase regulates the Par protein complex activity to control cell polarization
- PMID: 37635877
- PMCID: PMC10457011
- DOI: 10.3389/fcell.2023.1231489
Nox1-based NADPH oxidase regulates the Par protein complex activity to control cell polarization
Abstract
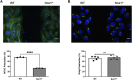
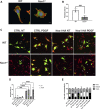
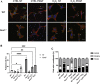
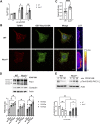
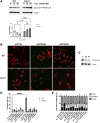
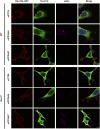
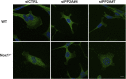
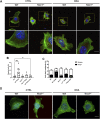
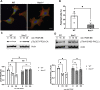
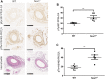
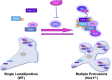
Cell migration is essential for many biological and pathological processes. Establishing cell polarity with a trailing edge and forming a single lamellipodium at the leading edge of the cell is crucial for efficient directional cell migration and is a hallmark of mesenchymal cell motility. Lamellipodia formation is regulated by spatial-temporal activation of the small GTPases Rac and Cdc42 at the front edge, and RhoA at the rear end. At a molecular level, partitioning-defective (Par) protein complex comprising Par3, Par6, and atypical Protein Kinase (aPKC isoforms ζ and λ/ι) regulates front-rear axis polarization. At the front edge, integrin clustering activates Cdc42, prompting the formation of Par3/Par6/aPKC complexes to modulate MTOC positioning and microtubule stabilization. Consequently, the Par3/Par6/aPKC complex recruits Rac1-GEF Tiam to activate Rac1, leading to lamellipodium formation. At the rear end, RhoA-ROCK phosphorylates Par3 disrupting its interaction with Tiam and inactivating Rac1. RhoA activity at the rear end allows the formation of focal adhesions and stress fibers necessary to generate the traction forces that allow cell movement. Nox1-based NADPH oxidase is necessary for PDGF-induced migration in vitro and in vivo for many cell types, including fibroblasts and smooth muscle cells. Here, we report that Nox1-deficient cells failed to acquire a normal front-to-rear polarity, polarize MTOC, and form a single lamellipodium. Instead, these cells form multiple protrusions that accumulate Par3 and active Tiam. The exogenous addition of H2O2 rescues this phenotype and is associated with the hyperactivation of Par3, Tiam, and Rac1. Mechanistically, Nox1 deficiency induces the inactivation of PP2A phosphatase, leading to increased activation of aPKC. These results were validated in Nox1y/- primary mouse aortic smooth muscle cells (MASMCs), which also showed PP2A inactivation after PDGF-BB stimulation consistent with exacerbated activation of aPKC. Moreover, we evaluated the physiological relevance of this signaling pathway using a femoral artery wire injury model to generate neointimal hyperplasia. Nox1y/- mice showed increased staining for the inactive form of PP2A and increased signal for active aPKC, suggesting that PP2A and aPKC activities might contribute to reducing neointima formation observed in the arteries of Nox1y/- mice.
Keywords: Nox1; PP2A; Par3; Polarity; migration.
Copyright © 2023 Valdivia, Duran, Lee, Williams, Lee and San Martin.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Structural Organization of Human Full-Length PAR3 and the aPKC-PAR6 Complex.Mol Biotechnol. 2022 Dec;64(12):1319-1327. doi: 10.1007/s12033-022-00504-1. Epub 2022 May 24. Mol Biotechnol. 2022. PMID: 35610404 Free PMC article.
-
PAR3-PAR6-atypical PKC polarity complex proteins in neuronal polarization.Cell Mol Life Sci. 2018 Aug;75(15):2735-2761. doi: 10.1007/s00018-018-2828-6. Epub 2018 Apr 25. Cell Mol Life Sci. 2018. PMID: 29696344 Free PMC article. Review.
-
Rho-kinase phosphorylates PAR-3 and disrupts PAR complex formation.Dev Cell. 2008 Feb;14(2):205-15. doi: 10.1016/j.devcel.2007.11.021. Dev Cell. 2008. PMID: 18267089
-
The Par-Tiam1 complex controls persistent migration by stabilizing microtubule-dependent front-rear polarity.Curr Biol. 2007 Oct 9;17(19):1623-34. doi: 10.1016/j.cub.2007.08.035. Epub 2007 Sep 6. Curr Biol. 2007. PMID: 17825562
-
The Roles of Par3, Par6, and aPKC Polarity Proteins in Normal Neurodevelopment and in Neurodegenerative and Neuropsychiatric Disorders.J Neurosci. 2022 Jun 15;42(24):4774-4793. doi: 10.1523/JNEUROSCI.0059-22.2022. J Neurosci. 2022. PMID: 35705493 Free PMC article. Review.
References
-
- Banai S., Wolf Y., Golomb G., Pearle A., Waltenberger J., Fishbein I., et al. (1998). PDGF-receptor tyrosine kinase blocker AG1295 selectively attenuates smooth muscle cell growth in vitro and reduces neointimal formation after balloon angioplasty in swine. Circulation 97 (19), 1960–1969. 10.1161/01.cir.97.19.1960 - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

