Dual targeting ovarian cancer by Muc16 CAR T cells secreting a bispecific T cell engager antibody for an intracellular tumor antigen WT1
- PMID: 37635172
- PMCID: PMC10991175
- DOI: 10.1007/s00262-023-03529-w
Dual targeting ovarian cancer by Muc16 CAR T cells secreting a bispecific T cell engager antibody for an intracellular tumor antigen WT1
Abstract
Epithelial ovarian cancer is the most lethal of gynecological cancers. The therapeutic efficacy of chimeric antigen receptor (CAR) T cell directed against single antigens is limited by the heterogeneous target antigen expression in epithelial ovarian tumors. To overcome this limitation, we describe an engineered cell with both dual targeting and orthogonal cytotoxic modalities directed against two tumor antigens that are highly expressed on ovarian cancer cells: cell surface Muc16 and intracellular WT1. Muc16-specific CAR T cells (4H11) were engineered to secrete a bispecific T cell engager (BiTE) constructed from a TCR mimic antibody (ESK1) reactive with the WT1-derived epitope RMFPNAPYL (RMF) presented by HLA-A2 molecules. The secreted ESK1 BiTE recruited and redirected other T cells to WT1 on the tumor cells. We show that ESK1 BiTE-secreting 4H11 CAR T cells exhibited enhanced anticancer activity against cancer cells with low Muc16 expression, compared to 4H11 CAR T cells alone, both in vitro and in mouse tumor models. Dual orthogonal cytotoxic modalities with different specificities targeting both surface and intracellular tumor-associated antigens present a promising strategy to overcome resistance to CAR T cell therapy in epithelial ovarian cancer and other cancers.
Keywords: Dual targeting; ESK1 BiTE; Muc16 CAR T cells; TCR mimic mAb; WT1.
© 2023. The Author(s), under exclusive licence to Springer-Verlag GmbH Germany, part of Springer Nature.
Conflict of interest statement
DAS is on a board of, has equity in, receives research funding from, has licensed technology with, or income from Lantheus, Sellas, Iovance, Arvinas, Clade, Pfizer, Actinium, Oncopep, Repertoire, Sapience, Atengen, CoImmune, and Eureka Therapeutics. TD is a consultant of Eureka Therapeutics. REO reports personal fees from Tesaro/GSK, Regeneron, Seattle Genetics, Fresenius Kabi, Gynecologic Oncology Foundation, Bayer, Curio/Onclive, R-Pharm, and Immunogen, other from Hitech Health, outside the submitted work. She is a non-compensated steering committee member for the PRIMA, Moonstone (Tesaro/GSK) and DUO-O (AstraZeneca) studies and non-compensated advisor for Carina Biotech. Her institute receives funding for clinical research from Bayer/Celgene/Juno, Tesaro/GSK, Merck, Ludwig Cancer Institute, Abbvie/StemCentrx, Regeneron, TCR2 Therapeutics, Atara Biotherapeutics, MarkerTherapeutics, Syndax Pharmaceuticals, Genmab/Seagen Therapeutics, Sellas Therapeutics, Genentech, Kite Pharma, and Gynecologic Oncology Foundation. LP is now an employee of Beam. TG is now an employee of Arsenal. All other authors declare no conflict or competing interests.
Figures
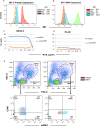
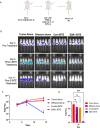

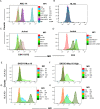
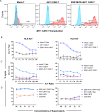
Update of
-
Dual targeting ovarian cancer by Muc16 CAR-T cells secreting a bispecific T cell engager antibody for an intracellular tumor antigen WT1.Res Sq [Preprint]. 2023 May 8:rs.3.rs-2887299. doi: 10.21203/rs.3.rs-2887299/v1. Res Sq. 2023. Update in: Cancer Immunol Immunother. 2023 Nov;72(11):3773-3786. doi: 10.1007/s00262-023-03529-w. PMID: 37214945 Free PMC article. Updated. Preprint.
Similar articles
-
Dual targeting ovarian cancer by Muc16 CAR-T cells secreting a bispecific T cell engager antibody for an intracellular tumor antigen WT1.Res Sq [Preprint]. 2023 May 8:rs.3.rs-2887299. doi: 10.21203/rs.3.rs-2887299/v1. Res Sq. 2023. Update in: Cancer Immunol Immunother. 2023 Nov;72(11):3773-3786. doi: 10.1007/s00262-023-03529-w. PMID: 37214945 Free PMC article. Updated. Preprint.
-
Therapeutic effect of dual CAR-T targeting PDL1 and MUC16 antigens on ovarian cancer cells in mice.BMC Cancer. 2020 Jul 20;20(1):678. doi: 10.1186/s12885-020-07180-x. BMC Cancer. 2020. PMID: 32689954 Free PMC article.
-
IL-12 secreting tumor-targeted chimeric antigen receptor T cells eradicate ovarian tumors in vivo.Oncoimmunology. 2015 Jan 23;4(3):e994446. doi: 10.4161/2162402X.2014.994446. eCollection 2015 Mar. Oncoimmunology. 2015. PMID: 25949921 Free PMC article.
-
Chimeric Antigen Receptor Design and Efficacy in Ovarian Cancer Treatment.Int J Mol Sci. 2021 Mar 28;22(7):3495. doi: 10.3390/ijms22073495. Int J Mol Sci. 2021. PMID: 33800608 Free PMC article. Review.
-
The challenge of selecting tumor antigens for chimeric antigen receptor T-cell therapy in ovarian cancer.Med Oncol. 2022 Sep 29;39(12):232. doi: 10.1007/s12032-022-01824-7. Med Oncol. 2022. PMID: 36175774 Review.
Cited by
-
Contemporary Approaches to Immunotherapy of Solid Tumors.Cancers (Basel). 2024 Jun 19;16(12):2270. doi: 10.3390/cancers16122270. Cancers (Basel). 2024. PMID: 38927974 Free PMC article. Review.
-
Recent advances in understanding the immune microenvironment in ovarian cancer.Front Immunol. 2024 Jun 5;15:1412328. doi: 10.3389/fimmu.2024.1412328. eCollection 2024. Front Immunol. 2024. PMID: 38903506 Free PMC article. Review.
-
A dual-targeting approach with anti-IL10R CAR-T cells engineered to release anti-CD33 bispecific antibody in enhancing killing effect on acute myeloid leukemia cells.Cell Oncol (Dordr). 2024 Oct;47(5):1879-1895. doi: 10.1007/s13402-024-00971-5. Epub 2024 Jul 15. Cell Oncol (Dordr). 2024. PMID: 39008193
-
MUC16: clinical targets with great potential.Clin Exp Med. 2024 May 17;24(1):101. doi: 10.1007/s10238-024-01365-5. Clin Exp Med. 2024. PMID: 38758220 Free PMC article. Review.
-
Structure-based design of antibodies targeting the EBNA1 DNA-binding domain to block Epstein-Barr virus latent infection and tumor growth.MedComm (2020). 2024 Oct 10;5(10):e739. doi: 10.1002/mco2.739. eCollection 2024 Oct. MedComm (2020). 2024. PMID: 39399647 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

