Prenatal and Neonatal Bone Health: Updated Review on Early Identification of Newborns at High Risk for Osteopenia
- PMID: 37630705
- PMCID: PMC10459154
- DOI: 10.3390/nu15163515
Prenatal and Neonatal Bone Health: Updated Review on Early Identification of Newborns at High Risk for Osteopenia
Abstract
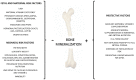
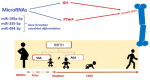
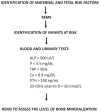
Bone health starts with maternal health and nutrition, which influences bone mass and density already in utero. The mechanisms underlying the effect of the intrauterine environment on bone health are partly unknown but certainly include the 'foetal programming' of oxidative stress and endocrine systems, which influence later skeletal growth and development. With this narrative review, we describe the current evidence for identifying patients with risk factors for developing osteopenia, today's management of these populations, and screening and prevention programs based on gestational age, weight, and morbidity. Challenges for bone health prevention include the need for new technologies that are specific and applicable to pregnant women, the foetus, and, later, the newborn. Radiofrequency ultrasound spectrometry (REMS) has proven to be a useful tool in the assessment of bone mineral density (BMD) in pregnant women. Few studies have reported that transmission ultrasound can also be used to assess BMD in newborns. The advantages of this technology in the foetus and newborn are the absence of ionising radiation, ease of use, and, above all, the possibility of performing longitudinal studies from intrauterine to extrauterine life. The use of these technologies already in the intrauterine period could help prevent associated diseases, such as osteoporosis and osteopenia, which are characterised by a reduction in bone mass and degeneration of bone structure and lead to an increased risk of fractures in adulthood with considerable social repercussions for the related direct and indirect costs.
Keywords: bone mineral density; endocrine disruptors; newborn infant; oxidative stress; ultrasounds.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
-
[Osteopenia of prematurity].Orv Hetil. 2005 Dec 4;146(49):2491-7. Orv Hetil. 2005. PMID: 16398314 Review. Hungarian.
-
Utilization of DXA Bone Mineral Densitometry in Ontario: An Evidence-Based Analysis.Ont Health Technol Assess Ser. 2006;6(20):1-180. Epub 2006 Nov 1. Ont Health Technol Assess Ser. 2006. PMID: 23074491 Free PMC article.
-
Screening for osteopenia and osteoporosis: selection by body composition.Osteoporos Int. 1996;6(2):120-6. doi: 10.1007/BF01623934. Osteoporos Int. 1996. PMID: 8704349
-
Systematic review and meta-analysis for the association of bone mineral density and osteoporosis/osteopenia with vascular calcification in women.Int J Rheum Dis. 2017 Feb;20(2):154-160. doi: 10.1111/1756-185X.12842. Epub 2016 May 6. Int J Rheum Dis. 2017. PMID: 27153243 Review.
Cited by
-
Osteoporosis and Bone Fragility in Children: Diagnostic and Treatment Strategies.J Clin Med. 2024 Aug 22;13(16):4951. doi: 10.3390/jcm13164951. J Clin Med. 2024. PMID: 39201093 Free PMC article. Review.
References
-
- Masztalerz-Kozubek D., Zielinska-Pukos M.A., Hamulka J. Maternal Diet, Nutritional Status, and Birth-Related Factors Influencing Offspring’s Bone Mineral Density: A Narrative Review of Observational, Cohort, and Randomized Controlled Trials. Nutrients. 2021;13:2302. doi: 10.3390/nu13072302. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

