The Proteome Profile of Olfactory Ecto-Mesenchymal Stem Cells-Derived from Patients with Familial Alzheimer's Disease Reveals New Insights for AD Study
- PMID: 37628788
- PMCID: PMC10454072
- DOI: 10.3390/ijms241612606
The Proteome Profile of Olfactory Ecto-Mesenchymal Stem Cells-Derived from Patients with Familial Alzheimer's Disease Reveals New Insights for AD Study
Abstract
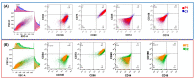
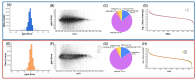
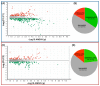
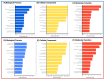
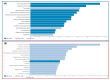
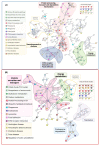
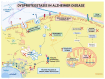
Alzheimer's disease (AD), the most common neurodegenerative disease and the first cause of dementia worldwide, has no effective treatment, and its pathological mechanisms are not yet fully understood. We conducted this study to explore the proteomic differences associated with Familial Alzheimer's Disease (FAD) in olfactory ecto-mesenchymal stem cells (MSCs) derived from PSEN1 (A431E) mutation carriers compared with healthy donors paired by age and gender through two label-free liquid chromatography-mass spectrometry approaches. The first analysis compared carrier 1 (patient with symptoms, P1) and its control (healthy donor, C1), and the second compared carrier 2 (patient with pre-symptoms, P2) with its respective control cells (C2) to evaluate whether the protein alterations presented in the symptomatic carrier were also present in the pre-symptom stages. Finally, we analyzed the differentially expressed proteins (DEPs) for biological and functional enrichment. These proteins showed impaired expression in a stage-dependent manner and are involved in energy metabolism, vesicle transport, actin cytoskeleton, cell proliferation, and proteostasis pathways, in line with previous AD reports. Our study is the first to conduct a proteomic analysis of MSCs from the Jalisco FAD patients in two stages of the disease (symptomatic and presymptomatic), showing these cells as a new and excellent in vitro model for future AD studies.
Keywords: A431E; Alzheimer’s disease; FAD; Familial Alzheimer’s disease; PSEN1; mesenchymal stem cells; neurodegeneration; olfactory; proteome; proteostasis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Olfactory Ecto-Mesenchymal Stem Cells in Modeling and Treating Alzheimer's Disease.Int J Mol Sci. 2024 Aug 3;25(15):8492. doi: 10.3390/ijms25158492. Int J Mol Sci. 2024. PMID: 39126059 Free PMC article. Review.
-
Mesenchymal Stem Cells from Familial Alzheimer's Patients Express MicroRNA Differently.Int J Mol Sci. 2024 Jan 27;25(3):1580. doi: 10.3390/ijms25031580. Int J Mol Sci. 2024. PMID: 38338859 Free PMC article.
-
Olfactory bulb proteome dynamics during the progression of sporadic Alzheimer's disease: identification of common and distinct olfactory targets across Alzheimer-related co-pathologies.Oncotarget. 2015 Nov 24;6(37):39437-56. doi: 10.18632/oncotarget.6254. Oncotarget. 2015. PMID: 26517091 Free PMC article.
-
Neurons derived from sporadic Alzheimer's disease iPSCs reveal elevated TAU hyperphosphorylation, increased amyloid levels, and GSK3B activation.Alzheimers Res Ther. 2017 Dec 1;9(1):90. doi: 10.1186/s13195-017-0317-z. Alzheimers Res Ther. 2017. PMID: 29191219 Free PMC article.
-
Genetic influences on white matter and metabolism abnormal change in Alzheimer's disease: Meta-analysis for neuroimaging research on presenilin 1 mutation.Clin Neurol Neurosurg. 2019 Feb;177:47-53. doi: 10.1016/j.clineuro.2018.12.016. Epub 2018 Dec 26. Clin Neurol Neurosurg. 2019. PMID: 30599314 Review.
Cited by
-
Olfactory Ecto-Mesenchymal Stem Cells in Modeling and Treating Alzheimer's Disease.Int J Mol Sci. 2024 Aug 3;25(15):8492. doi: 10.3390/ijms25158492. Int J Mol Sci. 2024. PMID: 39126059 Free PMC article. Review.
-
Proteoform Analysis of the Human Olfactory System: A Window into Neurodegenerative Diseases.Proteomes. 2024 Mar 21;12(1):9. doi: 10.3390/proteomes12010009. Proteomes. 2024. PMID: 38535507 Free PMC article. Review.
-
Mesenchymal Stem Cells from Familial Alzheimer's Patients Express MicroRNA Differently.Int J Mol Sci. 2024 Jan 27;25(3):1580. doi: 10.3390/ijms25031580. Int J Mol Sci. 2024. PMID: 38338859 Free PMC article.
-
Molecular Mechanism of Alzheimer's Disease.Int J Mol Sci. 2023 Nov 28;24(23):16837. doi: 10.3390/ijms242316837. Int J Mol Sci. 2023. PMID: 38069160 Free PMC article.
-
Circulating blood circular RNA in Parkinson's Disease; from involvement in pathology to diagnostic tools in at-risk individuals.NPJ Parkinsons Dis. 2024 Nov 18;10(1):222. doi: 10.1038/s41531-024-00839-3. NPJ Parkinsons Dis. 2024. PMID: 39557914 Free PMC article.
References
-
- Prince M.J. World Alzheimer Report 2015: The Global Impact of Dementia. 2015. [(accessed on 23 July 2020)]. Available online: https://www.alz.co.uk/research/world-report-2015.
-
- Nisbet R.M., Götz J. Amyloid-β and Tau in Alzheimer’s Disease: Novel Pathomechanisms and Non-Pharmacological Treatment Strategies. [(accessed on 23 July 2020)];J. Alzheimers Dis. 2018 64:S517–S527. doi: 10.3233/JAD-179907. Available online: https://www.medra.org/servlet/aliasResolver?alias=iospress&doi=10.3233/J.... - DOI - PubMed
-
- McKhann G.M., Knopman D.S., Chertkow H., Hymans B.T., Jack C.R., Kawas C.H., Klunk W.E., Koroshetz W.J., Manly J.J., Mayeux R., et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

