Hypoxic State of Cells and Immunosenescence: A Focus on the Role of the HIF Signaling Pathway
- PMID: 37626660
- PMCID: PMC10452839
- DOI: 10.3390/biomedicines11082163
Hypoxic State of Cells and Immunosenescence: A Focus on the Role of the HIF Signaling Pathway
Abstract
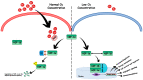
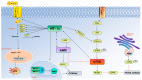
Hypoxia activates hypoxia-related signaling pathways controlled by hypoxia-inducible factors (HIFs). HIFs represent a quick and effective detection system involved in the cellular response to insufficient oxygen concentration. Activation of HIF signaling pathways is involved in improving the oxygen supply, promoting cell survival through anaerobic ATP generation, and adapting energy metabolism to meet cell demands. Hypoxia can also contribute to the development of the aging process, leading to aging-related degenerative diseases; among these, the aging of the immune system under hypoxic conditions can play a role in many different immune-mediated diseases. Thus, in this review we aim to discuss the role of HIF signaling pathways following cellular hypoxia and their effects on the mechanisms driving immune system senescence.
Keywords: cellular aging; cellular energy; hypoxia-inducible factors; immunosenescence.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Role of hypoxia in cellular senescence.Pharmacol Res. 2023 Aug;194:106841. doi: 10.1016/j.phrs.2023.106841. Epub 2023 Jun 28. Pharmacol Res. 2023. PMID: 37385572 Review.
-
Keeping the engine primed: HIF factors as key regulators of cardiac metabolism and angiogenesis during ischemia.J Mol Med (Berl). 2007 Dec;85(12):1309-15. doi: 10.1007/s00109-007-0279-x. Epub 2007 Nov 20. J Mol Med (Berl). 2007. PMID: 18026917 Review.
-
The Role of Hypoxia-Inducible Factor in the Mechanisms of Aging.Biochemistry (Mosc). 2022 Sep;87(9):995-1014. doi: 10.1134/S0006297922090115. Biochemistry (Mosc). 2022. PMID: 36180993 Review.
-
Hypoxia and aging.Exp Mol Med. 2019 Jun 20;51(6):1-15. doi: 10.1038/s12276-019-0233-3. Exp Mol Med. 2019. PMID: 31221957 Free PMC article. Review.
-
Cell metabolism under microenvironmental low oxygen tension levels in stemness, proliferation and pluripotency.Curr Mol Med. 2015;15(4):343-59. doi: 10.2174/1566524015666150505160406. Curr Mol Med. 2015. PMID: 25941818
Cited by
-
Dendritic Cells: A Bridge between Tolerance Induction and Cancer Development in Transplantation Setting.Biomedicines. 2024 Jun 3;12(6):1240. doi: 10.3390/biomedicines12061240. Biomedicines. 2024. PMID: 38927447 Free PMC article. Review.
-
mTOR and SGLT-2 Inhibitors: Their Synergistic Effect on Age-Related Processes.Int J Mol Sci. 2024 Aug 8;25(16):8676. doi: 10.3390/ijms25168676. Int J Mol Sci. 2024. PMID: 39201363 Free PMC article. Review.
-
Hypoxic Inducible Factor Stabilization in Pericytes beyond Erythropoietin Production: The Good and the Bad.Antioxidants (Basel). 2024 Apr 27;13(5):537. doi: 10.3390/antiox13050537. Antioxidants (Basel). 2024. PMID: 38790642 Free PMC article. Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

