In Vitro and In Vivo Evaluation of Epidermal Growth Factor (EGF) Loaded Alginate-Hyaluronic Acid (AlgHA) Microbeads System for Wound Healing
- PMID: 37623648
- PMCID: PMC10455903
- DOI: 10.3390/jfb14080403
In Vitro and In Vivo Evaluation of Epidermal Growth Factor (EGF) Loaded Alginate-Hyaluronic Acid (AlgHA) Microbeads System for Wound Healing
Abstract
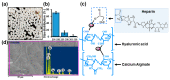
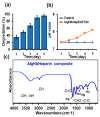
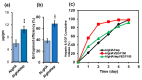
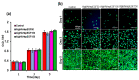
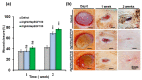
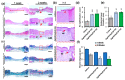
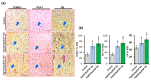
The management of skin injuries is one of the most common concerns in medical facilities. Different types of biomaterials with effective wound-healing characteristics have been studied previously. In this study, we used alginate (Alg) and hyaluronic acid (HA) composite (80:20) beads for the sustained release of epidermal growth factor (EGF) delivery. Heparin crosslinked AlgHA beads showed significant loading and entrapment of EGF. Encapsulated beads demonstrated biocompatibility with rat L929 cells and significant migration at the concentration of AlgHAEGF100 and AlgHAEGF150 within 24 h. Both groups significantly improved the expression of Fetal Liver Kinase 1 (FLK-1) along with the Intercellular Adhesion Molecule-1 (ICAM-1) protein in rat bone Mesenchymal stem cells (rbMSCs). In vivo assessment exhibited significant epithelialization and wound closure gaps within 2 weeks. Immunohistochemistry shows markedly significant levels of ICAM-1, FLK-1, and fibronectin (FN) in the AlgHAEGF100 and AlgHAEGF150 groups. Hence, we conclude that the EGF-loaded alginate-hyaluronic acid (AlgHA) bead system can be used to promote wound healing.
Keywords: alginate; epidermal growth factor; heparin; hyaluronic acid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Controlled release of vascular endothelial growth factor (VEGF) in alginate and hyaluronic acid (ALG-HA) bead system to promote wound healing in punch-induced wound rat model.J Biomater Sci Polym Ed. 2023 Apr;34(5):612-631. doi: 10.1080/09205063.2022.2135264. Epub 2022 Oct 18. J Biomater Sci Polym Ed. 2023. PMID: 36218190
-
Examination of In vitro and In vivo biocompatibility of alginate-hyaluronic acid microbeads As a promising method in cell delivery for kidney regeneration.Int J Biol Macromol. 2017 Dec;105(Pt 1):143-153. doi: 10.1016/j.ijbiomac.2017.07.019. Epub 2017 Jul 8. Int J Biol Macromol. 2017. PMID: 28698077
-
Hyaluronan/collagen hydrogels containing sulfated hyaluronan improve wound healing by sustained release of heparin-binding EGF-like growth factor.Acta Biomater. 2019 Mar 1;86:135-147. doi: 10.1016/j.actbio.2019.01.029. Epub 2019 Jan 17. Acta Biomater. 2019. PMID: 30660005
-
Development of a functional wound dressing composed of hyaluronic acid spongy sheet containing bioactive components: evaluation of wound healing potential in animal tests.J Biomater Sci Polym Ed. 2014;25(12):1278-91. doi: 10.1080/09205063.2014.929427. Epub 2014 Jun 24. J Biomater Sci Polym Ed. 2014. PMID: 24959914
-
Chitosan/Hyaluronic acid/Alginate and an assorted polymers loaded with honey, plant, and marine compounds for progressive wound healing-Know-how.Int J Biol Macromol. 2021 Sep 1;186:656-685. doi: 10.1016/j.ijbiomac.2021.07.067. Epub 2021 Jul 14. Int J Biol Macromol. 2021. PMID: 34271047 Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

