Exploratory analysis to predict pneumonitis during durvalumab consolidation therapy for patients with locally advanced non-small cell lung cancer from proteomic profiling of circulating extracellular vesicles
- PMID: 37614219
- PMCID: PMC10569905
- DOI: 10.1111/1759-7714.15077
Exploratory analysis to predict pneumonitis during durvalumab consolidation therapy for patients with locally advanced non-small cell lung cancer from proteomic profiling of circulating extracellular vesicles
Abstract
Background: Risk factors for predicting pneumonitis during durvalumab consolidation after chemoradiotherapy (CRT) in locally advanced non-small cell lung cancer (LA-NSCLC) are still lacking. Extracellular vesicles (EVs) play a crucial role in intercellular communication and are potential diagnostic tools for various diseases.
Methods: We retrospectively collected predurvalumab treatment serum samples from patients treated with durvalumab for LA-NSCLC, isolated EVs using anti-CD9 and anti-CD63 antibodies, and performed proteomic analyses. We examined EV proteins that could predict the development of symptomatic pneumonitis (SP) during durvalumab treatment. Potential EV-protein biomarkers were validated in an independent cohort.
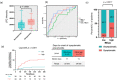
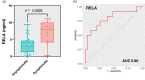
Results: In the discovery cohort, 73 patients were included, 49 with asymptomatic pneumonitis (AP) and 24 with SP. Of the 5797 proteins detected in circulating EVs, 33 were significantly elevated (fold change [FC] > 1.5, p < 0.05) in the SP group, indicating enrichment of the nuclear factor kappa B (NF-κB) pathway. Patients with high levels of EV-RELA, an NF-κB subunit, had a higher incidence of SP than those with low levels of EV-RELA (53.8% vs. 13.4%, p = 0.0017). In the receiver operating characteristic analysis, EV-RELA demonstrated a higher area under the curve (AUC) than lung V20 (0.76 vs. 0.62) and was identified as an independent risk factor in the multivariate logistic regression analysis (p = 0.008, odds ratio 7.72). Moreover, high EV-RELA was also a predictor of SP in the validation cohort comprising 43 patients (AUC of 0.80).
Conclusions: Circulating EV-RELA may be a predictive marker for symptomatic pneumonitis in patients with LA-NSCLC treated with durvalumab.
Keywords: chemoradiotherapy; durvalumab; extracellular vesicles; pneumonitis.
© 2023 The Authors. Thoracic Cancer published by China Lung Oncology Group and John Wiley & Sons Australia, Ltd.
Conflict of interest statement
Dr Torasawa has nothing to disclose. Dr. Horinouchi reports receiving personal fees from AstraZeneca.K.K during the conduct of the study; grants and personal fees from Chugai, Merck Sharp & Dohme, Novartis, Ono, and Roche; grants from AbbVie, Bristol‐Myers Squibb, Daiichi Sankyo, Genomic Health, Janssen, Merck Biopharma; and personal fees from Eli Lilly and Kyowa‐Kirin, outside of the submitted work. Dr Yagishita reports receiving grants from Nippon Boehringer Ingelheim, outside of the submitted work. Dr Utsumi has nothing to disclose. Dr Okuda has nothing to disclose. Dr Takekoshi has nothing to disclose. Dr Ito has nothing to disclose. Dr Wakui reports receiving personal fees from AstraZeneca.K.K during the conduct of the study.
Dr Murata has nothing to disclose. Dr Kaku has nothing to disclose. Dr Okuma has nothing to disclose. Dr Sinnno reports receiving personal fees from AstraZeneca.K.K during the conduct of the study; personal fees from Bristol‐Myers Squibb, Chugai, and Eli Lilly; grants and personal fees from Ono; and grants from Janssen and Japan Clinical Research Operations K.K. outside of the submitted work. Dr Matsumoto reports receiving personal fees from AstraZeneca.K.K during the conduct of the study; grants from Grant‐in‐Aid for Scientific Research on Innovative Areas, Hitachi, Ltd., and the National Cancer Center Research and Development Fund; grants and personal fees from Olympus; and personal fees from AMCO Inc., Chugai, COOK, Eli Lilly, Erbe Elektromedizin GmbH, Fujifilm, Novartis, and Thermo Fisher Scientific outside of the submitted work. Dr Okuma reports receiving personal fees from AstraZeneca.K.K during the conduct of the study; grants from AbbVie K.K. and Roche; and personal fees from Nippon Boehringer Ingelheim, Bristol‐Myers Squibb, Chugai Pharma Co. Ltd., Ely Lilly K.K., Ono Pharma Co. Ltd., Pfizer Taiho Pharma Co. Ltd., and Taiho Pharma Co. Ltd. outside of the submitted work. Dr Yoshida reports receiving personal fees from AstraZeneca.K.K during the conduct of the study; grants and personal fees from Amgen, Bristol‐Myers Squibb, Chugai, Merck Sharp & Dohme, Novartis, and Ono; grants from AbbVie, Daiichi Sankyo, and Takeda; and personal fees from ArcherDX, Eli Lilly, Roche, and Taiho outside of the submitted work. Dr Goto reports receiving personal fees from AstraZeneca.K.K during the conduct of the study; grants from AbbVie, AZK, Kyorin, and Preferred Network; grants and personal fees from Bristol‐Myers Squibb, Daiichi Sankyo, Eli Lilly, Novartis, Ono, and Pfizer; and personal fees from Boehringer Ingelheim, Chugai, Guardant Health Inc., Illumina, Merck Sharp & Dohme, Taiho, and Thermo Fisher outside of the submitted work. Dr. Yamamoto reports receiving personal fees from AstraZeneca.K.K during the conduct of the study; grants from Astellas, Bayer, Boehringer Ingelheim, Bristol‐Myers Squibb, Carna Biosciences, Chugai, Chiome Bioscience Inc., Daiichi Sankyo, Eisai, Eli Lilly, Genmab, GlaxoSmithKline, Janssen Pharma, Kyowa‐Hakko Kirin, Merck Sharp & Dohme, Novartis, Ono Pharmaceutical Co., Ltd., Otsuka, Pfizer, Quintiles, Shionogi, Sumitomo Dainippon, Takeda, and Taiho; and personal fees from Boehringer Ingelheim, Bristol‐Myers Squibb, Chugai, Cimic, Eisai, Lilly, Ono Pharmaceutical Co., Ltd., Otsuka, Pfizer, Sysmex, and Takeda, outside of the submitted work. Dr Araya receiving grants from Japanese Respiratory Foundation and Kowa company, LTD (campany x) outside of the submitted work. Dr Ohe reports receiving grants, personal fees, and nonfinancial support from AstraZeneca.K.K during the conduct of the study; personal fees from Amgen, AnHeeart Therapeutics Inc., Bayer, Boehringer Ingelheim, Bristol‐Myers Squibb, Celltrion, Chugai, Kyowa‐Hakko Kirin, Merck Sharp & Dohme, Nippon Kayaku, Ono Pharmaceutical Co., Ltd., Pfizer, and Taiho; grants and personal fees from Eli Lilly; grants and nonfinancial support from Kyorin; grants from Daiichi Sankyo, Dainippon‐Sumitomo, Janssen, Kissei, LOXO, Novartis, Takeda, and Taiho, outside of the submitted work. Dr Fujita reports receiving grants from AstraZeneca.K.K during the conduct of the study; grants from Preferred networks, Showa Denko Materials Co. Ltd., SHIBUYA Cooperation, H.U. Group Holdings, Inc., Merck Sharp & Dohme outside of the submitted work.
Figures


Similar articles
-
Predicting factors of symptomatic radiation pneumonitis induced by durvalumab following concurrent chemoradiotherapy in locally advanced non-small cell lung cancer.Radiat Oncol. 2022 Jan 15;17(1):7. doi: 10.1186/s13014-021-01979-z. Radiat Oncol. 2022. PMID: 35033139 Free PMC article.
-
Real-world survey of pneumonitis and its impact on durvalumab consolidation therapy in patients with non-small cell lung cancer who received chemoradiotherapy after durvalumab approval (HOPE-005/CRIMSON).Lung Cancer. 2021 Nov;161:86-93. doi: 10.1016/j.lungcan.2021.08.019. Epub 2021 Sep 5. Lung Cancer. 2021. PMID: 34543942
-
Chemoradiotherapy followed by durvalumab in patients with unresectable advanced non-small cell lung cancer: Management of adverse events.Thorac Cancer. 2020 May;11(5):1280-1287. doi: 10.1111/1759-7714.13394. Epub 2020 Mar 11. Thorac Cancer. 2020. PMID: 32160383 Free PMC article.
-
Pneumonitis Risk After Chemoradiotherapy With and Without Immunotherapy in Patients With Locally Advanced Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis.Int J Radiat Oncol Biol Phys. 2024 Jul 15;119(4):1179-1207. doi: 10.1016/j.ijrobp.2024.01.217. Epub 2024 Feb 13. Int J Radiat Oncol Biol Phys. 2024. PMID: 38360117 Review.
-
Immune-Related Pneumonitis After Chemoradiotherapy and Subsequent Immune Checkpoint Blockade in Unresectable Stage III Non-Small-Cell Lung Cancer.Clin Lung Cancer. 2020 Sep;21(5):e435-e444. doi: 10.1016/j.cllc.2020.02.025. Epub 2020 Mar 9. Clin Lung Cancer. 2020. PMID: 32576443 Review.
References
-
- Goldstraw P, Chansky K, Crowley J, Rami‐Porta R, Asamura H, Eberhardt WEE, et al. The IASLC lung cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol. 2016;11(1):39–51. 10.1016/j.jtho.2015.09.009 - DOI - PubMed
-
- Zenke Y, Tsuboi M, Chiba Y, Tsujino K, Satouchi M, Sawa K, et al. Effect of second‐generation vs third‐generation chemotherapy regimens with thoracic radiotherapy on Unresectable stage III non–small‐cell lung cancer: 10‐year follow‐up of a WJTOG0105 phase 3 randomized clinical trial. JAMA Oncol. 2021;7(6):904–909. 10.1001/jamaoncol.2021.0113 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

