C1GalT1 expression reciprocally controls tumour cell-cell and tumour-macrophage interactions mediated by galectin-3 and MGL with double impact on cancer development and progression
- PMID: 37612278
- PMCID: PMC10447578
- DOI: 10.1038/s41419-023-06082-7
C1GalT1 expression reciprocally controls tumour cell-cell and tumour-macrophage interactions mediated by galectin-3 and MGL with double impact on cancer development and progression
Abstract
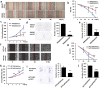
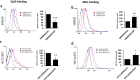
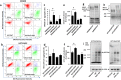
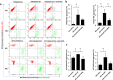
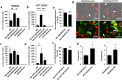
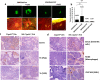
Although most cell membrane proteins are modified by glycosylation, our understanding of the role and actions of protein glycosylation is still very limited. β1,3galactosyltransferase (C1GalT1) is a key glycosyltransferase that controls the biosynthesis of the Core 1 structure of O-linked mucin type glycans and is overexpressed by many common types of epithelial cancers. This study reports that suppression of C1GalT1 expression in human colon cancer cells caused substantial changes of protein glycosylation of cell membrane proteins, many of which were ligands of the galactoside-binding galectin-3 and the macrophage galactose-type lectin (MGL). This led to significant reduction of cancer cell proliferation, adhesion, migration and the ability of tumour cells to form colonies. Crucially, C1GalT1 suppression significantly reduced galectin-3-mediated tumour cell-cell interaction and galectin-3-promoted tumour cell activities. In the meantime, C1GalT1 suppression substantially increased MGL-mediated macrophage-tumour cell interaction and macrophage-tumour cell phagocytosis and cytokine secretion. C1GalT1-expressing cancer cells implanted in chick embryos resulted in the formation of significantly bigger tumours than C1GalT1-suppressed cells and the presence of galectin-3 increased tumour growth of C1GalT1-expressing but not C1GalT1-suppressed cells. More MGL-expressing macrophages and dendritic cells were seen to be attracted to the tumour microenvironment in ME C1galt1-/-/Erb mice than in C1galt1f/f /Erb mice. These results indicate that expression of C1GalT1 by tumour cells reciprocally controls tumour cell-cell and tumour-macrophage interactions mediated by galectin-3 and MGL with double impact on cancer development and progression. C1GalT1 overexpression in epithelial cancers therefore may represent a fundamental mechanism in cancer promotion and in reduction of immune response/surveillance in cancer progression.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Expression and Impact of C1GalT1 in Cancer Development and Progression.Cancers (Basel). 2021 Dec 15;13(24):6305. doi: 10.3390/cancers13246305. Cancers (Basel). 2021. PMID: 34944925 Free PMC article. Review.
-
C1GALT1 overexpression promotes the invasive behavior of colon cancer cells through modifying O-glycosylation of FGFR2.Oncotarget. 2014 Apr 30;5(8):2096-106. doi: 10.18632/oncotarget.1815. Oncotarget. 2014. PMID: 24758762 Free PMC article.
-
Differential glycosylation of MUC1 and CEACAM5 between normal mucosa and tumour tissue of colon cancer patients.Int J Cancer. 2012 Jul 1;131(1):117-28. doi: 10.1002/ijc.26354. Epub 2011 Nov 28. Int J Cancer. 2012. PMID: 21823122
-
O-Glycosylation-mediated signaling circuit drives metastatic castration-resistant prostate cancer.FASEB J. 2018 Jun 15:fj201800687. doi: 10.1096/fj.201800687. Online ahead of print. FASEB J. 2018. PMID: 29906246
-
C1GALT1 in health and disease.Oncol Lett. 2021 Aug;22(2):589. doi: 10.3892/ol.2021.12850. Epub 2021 Jun 6. Oncol Lett. 2021. PMID: 34149900 Free PMC article. Review.
Cited by
-
High core 1β1,3-galactosyltransferase 1 expression is associated with poor prognosis and promotes cellular radioresistance in lung adenocarcinoma.J Cancer Res Clin Oncol. 2024 Apr 25;150(4):214. doi: 10.1007/s00432-024-05745-y. J Cancer Res Clin Oncol. 2024. PMID: 38662050 Free PMC article.
-
C1GALT1 induces the carcinogenesis of thyroid cancer through regulation by miR-141-3p and GLUT1.Heliyon. 2024 May 24;10(11):e31778. doi: 10.1016/j.heliyon.2024.e31778. eCollection 2024 Jun 15. Heliyon. 2024. PMID: 38845937 Free PMC article.
-
Unraveling the role of C1GALT1 in abnormal glycosylation and colorectal cancer progression.Front Oncol. 2024 Apr 18;14:1389713. doi: 10.3389/fonc.2024.1389713. eCollection 2024. Front Oncol. 2024. PMID: 38699634 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

