Plasticity of face-hand sensorimotor circuits after a traumatic brachial plexus injury
- PMID: 37609451
- PMCID: PMC10440702
- DOI: 10.3389/fnins.2023.1221777
Plasticity of face-hand sensorimotor circuits after a traumatic brachial plexus injury
Abstract
Background: Interactions between the somatosensory and motor cortices are of fundamental importance for motor control. Although physically distant, face and hand representations are side by side in the sensorimotor cortex and interact functionally. Traumatic brachial plexus injury (TBPI) interferes with upper limb sensorimotor function, causes bilateral cortical reorganization, and is associated with chronic pain. Thus, TBPI may affect sensorimotor interactions between face and hand representations.
Objective: The aim of this study was to investigate changes in hand-hand and face-hand sensorimotor integration in TBPI patients using an afferent inhibition (AI) paradigm.
Method: The experimental design consisted of electrical stimulation (ES) applied to the hand or face followed by transcranial magnetic stimulation (TMS) to the primary motor cortex to activate a hand muscle representation. In the AI paradigm, the motor evoked potential (MEP) in a target muscle is significantly reduced when preceded by an ES at short-latency (SAI) or long-latency (LAI) interstimulus intervals. We tested 18 healthy adults (control group, CG), evaluated on the dominant upper limb, and nine TBPI patients, evaluated on the injured or the uninjured limb. A detailed clinical evaluation complemented the physiological investigation.
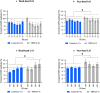
Results: Although hand-hand SAI was present in both the CG and the TBPI groups, hand-hand LAI was present in the CG only. Moreover, less AI was observed in TBPI patients than the CG both for face-hand SAI and LAI.
Conclusion: Our results indicate that sensorimotor integration involving both hand and face sensorimotor representations is affected by TBPI.
Keywords: afferent inhibition; brachial plexus lesion; corticospinal excitability; deafferentation; pain; transcranial magnetic stimulation.
Copyright © 2023 Torres, Ramalho, Rodrigues, Schmaedeke, Moraes, Reilly, Carvalho and Vargas.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Role of cutaneous and proprioceptive inputs in sensorimotor integration and plasticity occurring in the facial primary motor cortex.J Physiol. 2020 Feb;598(4):839-851. doi: 10.1113/JP278877. Epub 2020 Feb 3. J Physiol. 2020. PMID: 31876950
-
Kinematic Changes in the Uninjured Limb After a Traumatic Brachial Plexus Injury.Front Hum Neurosci. 2021 Dec 9;15:777776. doi: 10.3389/fnhum.2021.777776. eCollection 2021. Front Hum Neurosci. 2021. PMID: 34955793 Free PMC article.
-
The recent history of afferent stimulation modulates corticospinal excitability.Neuroimage. 2022 Sep;258:119365. doi: 10.1016/j.neuroimage.2022.119365. Epub 2022 Jun 9. Neuroimage. 2022. PMID: 35690256
-
Short- and long-latency afferent inhibition; uses, mechanisms and influencing factors.Brain Stimul. 2018 Jan-Feb;11(1):59-74. doi: 10.1016/j.brs.2017.09.009. Epub 2017 Sep 20. Brain Stimul. 2018. PMID: 28964754 Review.
-
Integrated technology for evaluation of brain function and neural plasticity.Phys Med Rehabil Clin N Am. 2004 Feb;15(1):263-306. doi: 10.1016/s1047-9651(03)00124-4. Phys Med Rehabil Clin N Am. 2004. PMID: 15029909 Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources

