Evaluation of the antibacterial activity of Elsholtzia ciliate essential oil against halitosis-related Fusobacterium nucleatum and Porphyromonas gingivalis
- PMID: 37608950
- PMCID: PMC10440386
- DOI: 10.3389/fmicb.2023.1219004
Evaluation of the antibacterial activity of Elsholtzia ciliate essential oil against halitosis-related Fusobacterium nucleatum and Porphyromonas gingivalis
Abstract
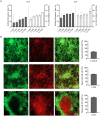
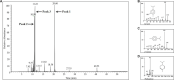
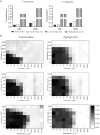
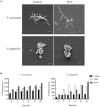
The broad-spectrum antimicrobial activity of Elsholtzia ciliate essential oil (ECO) has been previously reported, but its effectiveness against halitosis-causing bacteria such as Fusobacterium nucleatum and Porphyromonas gingivalis is not well understood. In this study, we investigated the bacteriostatic activity of ECO against planktonic cells and biofilms of F. nucleatum and P. gingivalis, as well as its ability to inhibit bacterial metabolism and production of volatile sulfur compounds (VSCs) at sub-lethal concentrations. Our findings revealed that ECO exhibited comparable activities to chlorhexidine against these oral bacteria. Treatment with ECO significantly reduced the production of VSCs, including hydrogen sulfide, dimethyl disulfide, and methanethiol, which are major contributors to bad breath. As the major chemical components of ECO, carvacrol, p-cymene, and phellandrene, were demonstrated in vitro inhibitory effects on F. nucleatum and P. gingivalis, and their combined use showed synergistic and additive effects, suggesting that the overall activity of ECO is derived from the cumulative or synergistic effect of multiple active components. ECO was found to have a destructive effect on the bacterial cell membrane by examining the cell morphology and permeability. Furthermore, the application of ECO induced significant changes in the bacterial composition of saliva-derived biofilm, resulting in the elimination of bacterial species that contribute to halitosis, including Fusobacterium, Porphyromonas, and Prevotella. These results provide experimental evidence for the potential clinical applications of ECOs in the prevention and treatment of halitosis.
Keywords: Elsholtzia ciliate; Fusobacterium nucleatum; Porphyromonas gingivalis; antibacterial activity; biofilm; essential oil; halitosis.
Copyright © 2023 Li, Wang, Xu, Wang, Cao, Wang, Zhang, Xu, Wang, Pan and Hu.
Conflict of interest statement
JX was employed by Shenzhen RELX Technology Company Limited. MC, SW, and TZ were employed by Shandong Aobo Biotechnology Company Limited. YX was employed by Beijing Xinyue Technology Company Limited. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Figures


Similar articles
-
Halitosis vaccines targeting FomA, a biofilm-bridging protein of fusobacteria nucleatum.Curr Mol Med. 2013 Sep;13(8):1358-67. doi: 10.2174/15665240113139990063. Curr Mol Med. 2013. PMID: 23865430 Review.
-
Inhibition of malodorous gas formation by oral bacteria with cetylpyridinium and zinc chloride.Arch Oral Biol. 2017 Dec;84:133-138. doi: 10.1016/j.archoralbio.2017.09.023. Epub 2017 Sep 28. Arch Oral Biol. 2017. PMID: 28987726
-
Lavandula angustifolia Essential Oil Inhibits the Ability of Fusobacterium nucleatum to Produce Volatile Sulfide Compounds, a Key Components in Oral Malodor.Molecules. 2024 Jun 23;29(13):2982. doi: 10.3390/molecules29132982. Molecules. 2024. PMID: 38998934 Free PMC article.
-
Label-free quantitative proteomic analysis of the oral bacteria Fusobacterium nucleatum and Porphyromonas gingivalis to identify protein features relevant in biofilm formation.Anaerobe. 2021 Dec;72:102449. doi: 10.1016/j.anaerobe.2021.102449. Epub 2021 Sep 17. Anaerobe. 2021. PMID: 34543761
-
The Role of Oral Microbiota in Intra-Oral Halitosis.J Clin Med. 2020 Aug 2;9(8):2484. doi: 10.3390/jcm9082484. J Clin Med. 2020. PMID: 32748883 Free PMC article. Review.
Cited by
-
Antimicrobial Effects of Edible Mixed Herbal Extracts on Oral Microorganisms: An In Vitro Study.Medicina (Kaunas). 2023 Oct 4;59(10):1771. doi: 10.3390/medicina59101771. Medicina (Kaunas). 2023. PMID: 37893489 Free PMC article.
-
Efficacy of a mouthwash containing ε-poly-L-lysine, funme peptides and domiphen in reducing halitosis and supragingival plaque: a randomized clinical trial.BMC Oral Health. 2024 May 3;24(1):525. doi: 10.1186/s12903-024-04255-0. BMC Oral Health. 2024. PMID: 38702623 Free PMC article. Clinical Trial.
-
Genetic components of Escherichia coli involved in its complex prey-predator interaction with Myxococcus xanthus.Front Microbiol. 2023 Dec 5;14:1304874. doi: 10.3389/fmicb.2023.1304874. eCollection 2023. Front Microbiol. 2023. PMID: 38116529 Free PMC article.
-
Acute and Sub-Chronic Intraperitoneal Toxicity Studies of the Elsholtzia ciliata Herbal Extract in Balb/c Mice.Pharmaceutics. 2023 Oct 3;15(10):2417. doi: 10.3390/pharmaceutics15102417. Pharmaceutics. 2023. PMID: 37896177 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials

