Primate TRIM34 is a broadly-acting, TRIM5-dependent lentiviral restriction factor
- PMID: 37608289
- PMCID: PMC10464172
- DOI: 10.1186/s12977-023-00629-4
Primate TRIM34 is a broadly-acting, TRIM5-dependent lentiviral restriction factor
Abstract
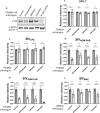
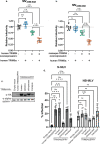
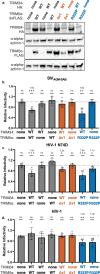
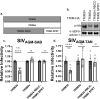
Human immunodeficiency virus (HIV) and other lentiviruses adapt to new hosts by evolving to evade host-specific innate immune proteins that differ in sequence and often viral recognition between host species. Understanding how these host antiviral proteins, called restriction factors, constrain lentivirus replication and transmission is key to understanding the emergence of pandemic viruses like HIV-1. Human TRIM34, a paralogue of the well-characterized lentiviral restriction factor TRIM5α, was previously identified by our lab via CRISPR-Cas9 screening as a restriction factor of certain HIV and SIV capsids. Here, we show that diverse primate TRIM34 orthologues from non-human primates can restrict a range of Simian Immunodeficiency Virus (SIV) capsids including SIVAGM-SAB, SIVAGM-TAN and SIVMAC capsids, which infect sabaeus monkeys, tantalus monkeys, and rhesus macaques, respectively. All primate TRIM34 orthologues tested, regardless of species of origin, were able to restrict this same subset of viral capsids. However, in all cases, this restriction also required the presence of TRIM5α. We demonstrate that TRIM5α is necessary, but not sufficient, for restriction of these capsids, and that human TRIM5α functionally interacts with TRIM34 from different species. Finally, we find that both the TRIM5α SPRY v1 loop and the TRIM34 SPRY domain are essential for TRIM34-mediated restriction. These data support a model in which TRIM34 is a broadly-conserved primate lentiviral restriction factor that acts in tandem with TRIM5α, such that together, these proteins can restrict capsids that neither can restrict alone.
© 2023. Diane D. Jeang.
Conflict of interest statement
The authors declare no competing interests.
Figures
Update of
-
Primate TRIM34 is a broadly-acting, TRIM5-dependent lentiviral restriction factor.bioRxiv [Preprint]. 2023 Mar 25:2023.03.24.534139. doi: 10.1101/2023.03.24.534139. bioRxiv. 2023. Update in: Retrovirology. 2023 Aug 22;20(1):15. doi: 10.1186/s12977-023-00629-4 PMID: 36993223 Free PMC article. Updated. Preprint.
Similar articles
-
Primate TRIM34 is a broadly-acting, TRIM5-dependent lentiviral restriction factor.bioRxiv [Preprint]. 2023 Mar 25:2023.03.24.534139. doi: 10.1101/2023.03.24.534139. bioRxiv. 2023. Update in: Retrovirology. 2023 Aug 22;20(1):15. doi: 10.1186/s12977-023-00629-4 PMID: 36993223 Free PMC article. Updated. Preprint.
-
TRIM34 restricts HIV-1 and SIV capsids in a TRIM5α-dependent manner.PLoS Pathog. 2020 Apr 13;16(4):e1008507. doi: 10.1371/journal.ppat.1008507. eCollection 2020 Apr. PLoS Pathog. 2020. PMID: 32282853 Free PMC article.
-
TRIM5alpha Modulates Immunodeficiency Virus Control in Rhesus Monkeys.PLoS Pathog. 2010 Jan 22;6(1):e1000738. doi: 10.1371/journal.ppat.1000738. PLoS Pathog. 2010. PMID: 20107597 Free PMC article.
-
SIVagm: genetic and biological features associated with replication.Front Biosci. 2003 Sep 1;8:d1170-85. doi: 10.2741/1130. Front Biosci. 2003. PMID: 12957815 Review.
-
Anti-retroviral activity of TRIM5 alpha.Rev Med Virol. 2010 Mar;20(2):77-92. doi: 10.1002/rmv.637. Rev Med Virol. 2010. PMID: 20049904 Review.
Cited by
-
TRIM5α: A Protean Architect of Viral Recognition and Innate Immunity.Viruses. 2024 Jun 21;16(7):997. doi: 10.3390/v16070997. Viruses. 2024. PMID: 39066160 Free PMC article. Review.
-
Virus specificity and nucleoporin requirements for MX2 activity are affected by GTPase function and capsid-CypA interactions.PLoS Pathog. 2024 Mar 21;20(3):e1011830. doi: 10.1371/journal.ppat.1011830. eCollection 2024 Mar. PLoS Pathog. 2024. PMID: 38512975 Free PMC article.
-
Capsid-dependent lentiviral restrictions.J Virol. 2024 Apr 16;98(4):e0030824. doi: 10.1128/jvi.00308-24. Epub 2024 Mar 18. J Virol. 2024. PMID: 38497663 Free PMC article. Review.
References
-
- Nisole S, Stoye JP, Saïb A. TRIM family proteins: retroviral restriction and antiviral defence. Nat Rev Microbiol. 2005;3:799–808. - PubMed
-
- Stremlau M, Owens CM, Perron MJ, Kiessling M, Autissier P, Sodroski J. The cytoplasmic body component TRIM5α restricts HIV-1 infection in Old World monkeys. Nature. 2004;427:848–53. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

