Combined Nano-Vector Mediated-Transfer to Suppress HIV-1 Infection with Targeted Antibodies in-vitro
- PMID: 37605734
- PMCID: PMC10440090
- DOI: 10.2147/IJN.S412915
Combined Nano-Vector Mediated-Transfer to Suppress HIV-1 Infection with Targeted Antibodies in-vitro
Abstract
Introduction: Broadly neutralizing antibodies (bNAbs) have the ability to neutralize a considerable breadth of genetically diverse human immunodeficiency virus (HIV) strains. Passive immunization can potentially provide protection against HIV infection in animal models. However, the direct antibody infusion effect is limited due to the short half-life and deficient immunogenicity of the antibody. As an alternative strategy, we propose the use of nano viral vectors, specifically the adeno-associated virus (AAV), to continuously and systematically produce bNAbs against HIV.
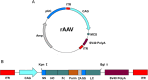
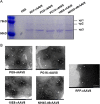
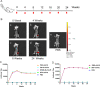
Methods: Plasmids expressing bNAbs PG9, PG16, 10E8, and NIH45-46 antibodies were constructed, targeting three different epitopes of HIV. Additionally, the bNAbs gene mediated by rAAV8 was administered to generate long-term expression with a single injection. We established both single and combined immunization groups. The neutralizing activity of antibodies expressed in mice sera was subsequently evaluated.
Results: The expression of bNAbs in BALB/c mice can last for >24 weeks after a single intramuscular injection of rAAV8. Further studies show that neutralization of the HIV pseudovirus by sera from co-immunized mice with rAAV8 expressing 10E8 and PG16 was enhanced compared with mice immunized with 10E8 or PG16 alone.
Conclusion: The prolonged expression of neutralizing antibodies can be maintained over long periods in BALB/c mice. This combined immunization is a promising candidate strategy for HIV treatment.
Keywords: AAV delivery; HIV therapy; combined immunotherapy; passive immunotherapy.
© 2023 Yao et al.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




Similar articles
-
Expression of HIV-1 broadly neutralizing antibodies mediated by recombinant adeno-associated virus 8 in vitro and in vivo.Mol Immunol. 2016 Dec;80:68-77. doi: 10.1016/j.molimm.2016.10.011. Epub 2016 Nov 12. Mol Immunol. 2016. PMID: 27835755
-
Broad and potent bispecific neutralizing antibody gene delivery using adeno-associated viral vectors for passive immunization against HIV-1.J Control Release. 2021 Oct 10;338:633-643. doi: 10.1016/j.jconrel.2021.09.006. Epub 2021 Sep 10. J Control Release. 2021. PMID: 34509584
-
Sensitivity to Broadly Neutralizing Antibodies of Recently Transmitted HIV-1 Clade CRF02_AG Viruses with a Focus on Evolution over Time.J Virol. 2019 Jan 4;93(2):e01492-18. doi: 10.1128/JVI.01492-18. Print 2019 Jan 15. J Virol. 2019. PMID: 30404804 Free PMC article.
-
Antibody gene transfer with adeno-associated viral vectors as a method for HIV prevention.Immunol Rev. 2017 Jan;275(1):324-333. doi: 10.1111/imr.12478. Immunol Rev. 2017. PMID: 28133808 Free PMC article. Review.
-
Adeno-Associated Viral Vector Mediated Expression of Broadly- Neutralizing Antibodies Against HIV-Hitting a Fast-Moving Target.Curr HIV Res. 2020;18(2):114-131. doi: 10.2174/1570162X18666200210121339. Curr HIV Res. 2020. PMID: 32039686 Review.
Cited by
-
Triple Combinations of AAV9-Vectors Encoding Anti-HIV bNAbs Provide Long-Term In Vivo Expression of Human IgG Effectively Neutralizing Pseudoviruses from HIV-1 Global Panel.Viruses. 2024 Aug 14;16(8):1296. doi: 10.3390/v16081296. Viruses. 2024. PMID: 39205270 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

