IFN-α induced systemic lupus erythematosus complicated with hemophagocytic lymphohistiocytosis: a case report and literature review
- PMID: 37600795
- PMCID: PMC10436618
- DOI: 10.3389/fimmu.2023.1223062
IFN-α induced systemic lupus erythematosus complicated with hemophagocytic lymphohistiocytosis: a case report and literature review
Abstract
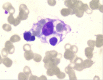
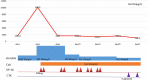
Hemophagocytic lymphohistiocytosis (HLH) is a severe and life-threatening hyperinflammatory condition characterized by excessive activation of macrophages and T cells and resulted in multi-organ dysfunction. HLH can be a primary disease or secondary to infections, malignancy, and some autoimmune diseases, including adult-onset Still's disease (AOSD) and systemic lupus erythematosus (SLE). However, it is rare for HLH to occur as a secondary condition to drug-induced lupus erythematosus (DILE). In this report, we present a case of HLH as an unusual complication during SLE treatment in a 31-year-old male patient. The patient initially suffered from active chronic hepatitis B (CHB) and was treated with pegylated INFα-2b (Peg-INFα-2b), tenofovir disoproxil and lamivudine. After 19 months, CHB obtained biochemical and virological response with HBsAg positive to HBsAb. The patient developed fever, headache, and cytopenia after Peg-INFα-2b treatment for 33 months, and laboratory studies revealed that ANA and anti dsDNA were positive. He displayed 5 features meeting the HLH-2004 criteria for diagnosis including fever, pancytopenia, hyperferritinemia, high levels of soluble CD25, and hemophagocytosis on bone marrow biopsy. The patient was initiated with a combination treatment of intravenous methylprednisolone pulse therapy, oral cyclosporine, and etoposide (VP-16), which was followed by a course of oral prednisolone, intravenous cyclophosphamide pulse therapy, and entecavir with complete response. To our knowledge, this is the first report of IFN-α induced SLE complicating with HLH. Physicians should consider the potential autoimmune side effects of IFN-α therapy and be alert to insidious HLH in patients diagnosed with SLE.
Keywords: Hemophagocytic lymphohistiocytosis (HLH); Peg-INFα-2b; hepatitis B virus (HBV); interferon-α; systemic lupus erythematosus (SLE).
Copyright © 2023 Zeng, Tu, Ji, Liu, Huang, Nie and Yang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Epstein-Barr Virus encephalitis associated hemophagocytic lymphohistiocytosis in childhood-onset systemic lupus erythematosus: a case-based review.Pediatr Rheumatol Online J. 2024 Oct 31;22(1):98. doi: 10.1186/s12969-024-01025-8. Pediatr Rheumatol Online J. 2024. PMID: 39482670 Free PMC article. Review.
-
Kikuchi-Fujimoto disease associated with systemic lupus erythematosus complicated with hemophagocytic lymphohistiocytosis: a case report.J Med Case Rep. 2019 Jun 6;13(1):173. doi: 10.1186/s13256-019-2100-1. J Med Case Rep. 2019. PMID: 31167644 Free PMC article.
-
An Unusual Presentation of Systemic Lupus Erythematosus as Hemophagocytic Lymphohistiocytosis in a Male.Cureus. 2019 Aug 19;11(8):e5427. doi: 10.7759/cureus.5427. Cureus. 2019. PMID: 31482047 Free PMC article.
-
Treatment of hemophagocytic lymphohistiocytosis with alemtuzumab in systemic lupus erythematosus.J Clin Rheumatol. 2012 Apr;18(3):134-7. doi: 10.1097/RHU.0b013e31824e8d9b. J Clin Rheumatol. 2012. PMID: 22426581
-
Acquired hemophagocytic lymphohistiocytosis as initial manifestation of multiple myeloma: A case report and literature review.Medicine (Baltimore). 2020 Sep 25;99(39):e22299. doi: 10.1097/MD.0000000000022299. Medicine (Baltimore). 2020. PMID: 32991435 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical