Effect of a smartphone intervention as a secondary prevention for use among university students with unhealthy alcohol use: randomised controlled trial
- PMID: 37586742
- PMCID: PMC10428135
- DOI: 10.1136/bmj-2022-073713
Effect of a smartphone intervention as a secondary prevention for use among university students with unhealthy alcohol use: randomised controlled trial
Abstract
Objective: To estimate the effects of providing access to an alcohol intervention based on a smartphone.
Design: Randomised controlled trial..
Setting: Four higher education institutions in Switzerland.
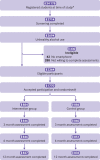
Participants: 1770 students (≥18 years) who screened positive for unhealthy alcohol use (ie, a score on the alcohol use disorders identification test-consumption (AUDIT-C) of ≥4 for men and ≥3 for women) were randomly assigned by 1:1 allocation ratio in blocks of 10.
Intervention: Providing access to a brief, smartphone based alcohol intervention.
Outcome measures: The primary outcome studied was number of standard drinks per week at six months and the secondary outcome was number of heavy drinking days (past 30 days). Additional outcomes were maximum number of drinks consumed on one occasion, alcohol related consequences, and academic performance. Follow-up assessments occurred at months three, six, and 12. Data were analysed by intention to treat and by using generalised linear mixed models with random intercepts for the recruitment site and participants nested within the recruitment site, and with intervention (v control), time (three months v six months; 12 months v six months), and baseline outcome values as fixed effects.
Results: Between 26 April 26 2021 and 30 May 2022, 1770 participants (intervention group (n=884); control group (n=886)) were included. Mean age was 22.4 years (standard deviation 3.07); 958 (54.1%) were women; and 1169 (66.0%) were undergraduate students, 533 (30.1%) were studying for a master's degree, 43 (2.4%) were studying for a doctorate, and 25 (1.4%) were students of other higher education programme. The baseline mean number of standard drinks per week was 8.59 (standard deviation 8.18); the baseline number of heavy drinking days was 3.53 (4.02). Of 1770 participants, follow-up rates were 1706 (96.4%) at three months, 1697 (95.9%) at six months, and 1660 (93.8%) at 12 months. Of 884 students randomly assigned to the intervention group, 738 (83.5%) downloaded the smartphone application. The intervention had a significant overall effect on the number of standard drinks per week (incidence rate ratio 0.90 (95% confidence interval 0.85 to 0.96)), heavy drinking days (0.89 (0.83 to 0.96)), and the maximum number of drinks consumed on one occasion (0.96 (0.93 to 1.00), P=0.029), indicating significantly lower drinking outcomes in the intervention group than in the control group during the follow-up period. The intervention did not affect alcohol related consequences or academic performance.
Conclusions: Providing access to the smartphone application throughout the 12 month follow-up was effective at limiting the average drinking volume of university students who had self-reported unhealthy alcohol use at baseline.
Trial registration: ISRCTN 10007691.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at http://www.icmje.org/disclosure-of-interest/ and declare: support from the Swiss National Science Foundation for the submitted work. NB reports a grant from: Commission de Promotion de la Santé et de Lutte Contre Les Addictions (CPSLA; Public regional funding from State of Vaud, Switzerland), as co-investigator; honoraria from the Swiss Medical Association, for the development of screening and brief intervention guidelines for primary care providers. AA reports a grant from CPSLA. GG reports a grant from the Swiss National Science Foundation (separate from the trial grant); he is a member of the technical advisory group on alcohol epidemiology, World Health Organization and of FEDESTAT (Federal commission on statistics, Swiss Federal Statistical Office, Berne, Switzerland). JAC reports grants from Canadian Institutes of Health Research (No. CFP 425862) as principal investigator and for a randomised controlled trial on targeting mailed nicotine patch distribution interventions to rural regions of Canada (Canadian Cancer Society, No. 706201, as principal investigator), and as a principal investigator for offering nicotine patches to all households in a municipality with high smoking rates (Canadian Institutes of Health Research, No. 153324). He was also involved in the trial: Does providing a brief Internet intervention for hazardous alcohol use to people seeking online help for depression reduce both alcohol use and depression symptoms among participants with these dual disorders? JM reports grants from NIH/NIDA, CDC, and a subcontract from the Northstar Node of the NIH/NIDA Clinical Trials Network. She also reports consulting fees from the National Committee for Quality Assurance, Expert for Alcohol Screening Learning Collaborative, Boston University, Fellowship Immersion Training, Faculty NYC Dept of Health and Mental Hygiene, buprenorphine mentorship program. She participates in data safety monitoring boards: as chair for extended-release v oral naltrexone for alcohol dependence treatment in primary care; as a member for integrated care for chronic pain and opioid use disorder: the IMPOWR Montefiore/Einstein (IMPOWR-ME) research center; and as a member for the randomised controlled trial of the effects of psilocybin-facilitated experience on the psychology and effectiveness of professional leaders in religion. She is a member of the Committee for the Care of Substance Users with HIV Infection, NYS Department of Health AIDS Institute, HIV Clinical Guidelines Program.
Figures
Similar articles
-
Smartphone-based secondary prevention intervention for university students with unhealthy alcohol use identified by screening: study protocol of a parallel group randomized controlled trial.Trials. 2020 Feb 17;21(1):191. doi: 10.1186/s13063-020-4145-2. Trials. 2020. PMID: 32066490 Free PMC article.
-
Web-based alcohol screening and brief intervention for university students: a randomized trial.JAMA. 2014 Mar 26;311(12):1218-24. doi: 10.1001/jama.2014.2138. JAMA. 2014. PMID: 24668103 Free PMC article. Clinical Trial.
-
Smartphone application for unhealthy alcohol use: Pilot randomized controlled trial in the general population.Drug Alcohol Depend. 2019 Feb 1;195:101-105. doi: 10.1016/j.drugalcdep.2018.12.002. Epub 2018 Dec 22. Drug Alcohol Depend. 2019. PMID: 30611977 Clinical Trial.
-
Social norms information for alcohol misuse in university and college students.Cochrane Database Syst Rev. 2015 Jan 26;1:CD006748. doi: 10.1002/14651858.CD006748.pub3. Cochrane Database Syst Rev. 2015. Update in: Cochrane Database Syst Rev. 2015 Dec 29;(12):CD006748. doi: 10.1002/14651858.CD006748.pub4. PMID: 25622306 Updated. Review.
-
Psychosocial interventions to reduce alcohol consumption in concurrent problem alcohol and illicit drug users.Cochrane Database Syst Rev. 2014 Dec 3;(12):CD009269. doi: 10.1002/14651858.CD009269.pub3. Cochrane Database Syst Rev. 2014. Update in: Cochrane Database Syst Rev. 2018 Dec 05;12:CD009269. doi: 10.1002/14651858.CD009269.pub4. PMID: 25470303 Updated. Review.
Cited by
-
Emergency transportation for acute alcohol intoxication four years after the coronavirus disease 2019 pandemic: a retrospective observational study.Environ Health Prev Med. 2024;29:53. doi: 10.1265/ehpm.24-00182. Environ Health Prev Med. 2024. PMID: 39384387 Free PMC article.
-
Randomized controlled trial of a smartphone app designed to reduce unhealthy alcohol consumption.Internet Interv. 2024 May 17;36:100747. doi: 10.1016/j.invent.2024.100747. eCollection 2024 Jun. Internet Interv. 2024. PMID: 38812955 Free PMC article.
-
Effectiveness of a smartphone app (Drink Less) versus usual digital care for reducing alcohol consumption among increasing-and-higher-risk adult drinkers in the UK: a two-arm, parallel-group, double-blind, randomised controlled trial.EClinicalMedicine. 2024 Mar 24;70:102534. doi: 10.1016/j.eclinm.2024.102534. eCollection 2024 Apr. EClinicalMedicine. 2024. PMID: 38685934 Free PMC article.
References
-
- Marmet S, Gmel G, Gmel G, Frick H, Rehm J. Alcohol-attributable mortality in Switzerland between 1997 and 2011. Addiction Suisse, 2013. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
