The atlastin ER morphogenic proteins promote formation of a membrane penetration site during non-enveloped virus entry
- PMID: 37578227
- PMCID: PMC10506488
- DOI: 10.1128/jvi.00756-23
The atlastin ER morphogenic proteins promote formation of a membrane penetration site during non-enveloped virus entry
Abstract
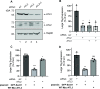
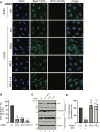
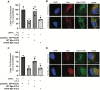
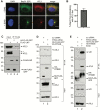
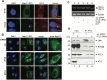
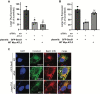
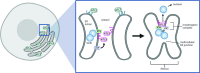
During entry, non-enveloped viruses penetrate a host membrane to cause infection, although how this is accomplished remains enigmatic. Polyomaviruses (PyVs) are non-enveloped DNA viruses that penetrate the endoplasmic reticulum (ER) membrane to reach the cytosol en route to the nucleus for infection. To penetrate the ER membrane, the prototype PyV simian virus 40 (SV40) induces formation of ER-escape sites, called foci, composed of repeating units of multi-tubular ER junctions where the virus is thought to exit. How SV40 triggers formation of the ER-foci harboring these multi-tubular ER junctions is unclear. Here, we show that the ER morphogenic atlastin 2 (ATL2) and ATL3 membrane proteins play critical roles in SV40 infection. Mechanistically, ATL3 mobilizes to the ER-foci where it deploys its GTPase-dependent membrane fusion activity to promote formation of multi-tubular ER junctions within the ER-foci. ATL3 also engages an SV40-containing membrane penetration complex. By contrast, ATL2 does not reorganize to the ER-foci. Instead, it supports the reticular ER morphology critical for the integrity of the ATL3-dependent membrane complex. Our findings illuminate how two host factors play distinct roles in the formation of an essential membrane penetration site for a non-enveloped virus. IMPORTANCE Membrane penetration by non-enveloped viruses, a critical infection step, remains enigmatic. The non-enveloped PyV simian virus 40 (SV40) penetrates the endoplasmic reticulum (ER) membrane to reach the cytosol en route for infection. During ER-to-cytosol membrane penetration, SV40 triggers formation of ER-associated structures (called ER-foci) that function as the membrane penetration sites. Here, we discover a role of the ATL ER membrane proteins-known to shape the ER morphology-during SV40-induced ER-foci formation. These findings illuminate how a non-enveloped virus hijacks host components to construct a membrane penetration structure.
Keywords: endoplasmic reticulum; entry; non-enveloped virus; simian virus 40.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
The endoplasmic reticulum membrane J protein C18 executes a distinct role in promoting simian virus 40 membrane penetration.J Virol. 2015 Apr;89(8):4058-68. doi: 10.1128/JVI.03574-14. Epub 2015 Jan 28. J Virol. 2015. PMID: 25631089 Free PMC article.
-
A nucleotide exchange factor promotes endoplasmic reticulum-to-cytosol membrane penetration of the nonenveloped virus simian virus 40.J Virol. 2015 Apr;89(8):4069-79. doi: 10.1128/JVI.03552-14. Epub 2015 Feb 4. J Virol. 2015. PMID: 25653441 Free PMC article.
-
Lunapark-dependent formation of a virus-induced ER exit site contains multi-tubular ER junctions that promote viral ER-to-cytosol escape.Cell Rep. 2021 Dec 7;37(10):110077. doi: 10.1016/j.celrep.2021.110077. Cell Rep. 2021. PMID: 34879280 Free PMC article.
-
SV40 Hijacks Cellular Transport, Membrane Penetration, and Disassembly Machineries to Promote Infection.Viruses. 2019 Oct 5;11(10):917. doi: 10.3390/v11100917. Viruses. 2019. PMID: 31590347 Free PMC article. Review.
-
How Polyomaviruses Exploit the ERAD Machinery to Cause Infection.Viruses. 2016 Aug 29;8(9):242. doi: 10.3390/v8090242. Viruses. 2016. PMID: 27589785 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

