Cancer stem cells in brain tumors: From origin to clinical implications
- PMID: 37576862
- PMCID: PMC10412776
- DOI: 10.1002/mco2.341
Cancer stem cells in brain tumors: From origin to clinical implications
Abstract
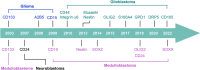
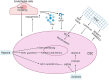
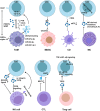
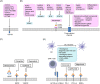
Malignant brain tumors are highly heterogeneous tumors with a poor prognosis and a high morbidity and mortality rate in both children and adults. The cancer stem cell (CSC, also named tumor-initiating cell) model states that tumor growth is driven by a subset of CSCs. This model explains some of the clinical observations of brain tumors, including the almost unavoidable tumor recurrence after initial successful chemotherapy and/or radiotherapy and treatment resistance. Over the past two decades, strategies for the identification and characterization of brain CSCs have improved significantly, supporting the design of new diagnostic and therapeutic strategies for brain tumors. Relevant studies have unveiled novel characteristics of CSCs in the brain, including their heterogeneity and distinctive immunobiology, which have provided opportunities for new research directions and potential therapeutic approaches. In this review, we summarize the current knowledge of CSCs markers and stemness regulators in brain tumors. We also comprehensively describe the influence of the CSCs niche and tumor microenvironment on brain tumor stemness, including interactions between CSCs and the immune system, and discuss the potential application of CSCs in brain-based therapies for the treatment of brain tumors.
Keywords: brain tumors; cancer stem cells; cell of origin; clinical implications; immune system; stemness regulators; targeted therapies; tumor microenvironment.
© 2023 The Authors. MedComm published by Sichuan International Medical Exchange & Promotion Association (SCIMEA) and John Wiley & Sons Australia, Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Cancer stem cells in hepatocellular carcinoma - from origin to clinical implications.Nat Rev Gastroenterol Hepatol. 2022 Jan;19(1):26-44. doi: 10.1038/s41575-021-00508-3. Epub 2021 Sep 9. Nat Rev Gastroenterol Hepatol. 2022. PMID: 34504325 Review.
-
Cancer Stem Cells in Hepatocellular Carcinoma: Intrinsic and Extrinsic Molecular Mechanisms in Stemness Regulation.Int J Mol Sci. 2022 Oct 14;23(20):12327. doi: 10.3390/ijms232012327. Int J Mol Sci. 2022. PMID: 36293184 Free PMC article. Review.
-
Cancer Stem Cells: Acquisition, Characteristics, Therapeutic Implications, Targeting Strategies and Future Prospects.Stem Cell Rev Rep. 2019 Jun;15(3):331-355. doi: 10.1007/s12015-019-09887-2. Stem Cell Rev Rep. 2019. PMID: 30993589 Review.
-
Targeting the Interplay Between Cancer Fibroblasts, Mesenchymal Stem Cells, and Cancer Stem Cells in Desmoplastic Cancers.Front Oncol. 2019 Jul 31;9:688. doi: 10.3389/fonc.2019.00688. eCollection 2019. Front Oncol. 2019. PMID: 31417869 Free PMC article. Review.
-
Evolving Strategies for Therapeutically Targeting Cancer Stem Cells.Adv Cancer Res. 2016;131:159-91. doi: 10.1016/bs.acr.2016.04.003. Epub 2016 Jun 3. Adv Cancer Res. 2016. PMID: 27451127 Review.
Cited by
-
Deciphering the role of transcription factors in glioblastoma cancer stem cells.Acta Biochim Biophys Sin (Shanghai). 2024 May 8;56(9):1245-1255. doi: 10.3724/abbs.2024061. Acta Biochim Biophys Sin (Shanghai). 2024. PMID: 38716541 Free PMC article. Review.
-
Effect of Apis mellifera syriaca Bee Venom on Glioblastoma Cancer: In Vitro and In Vivo Studies.Molecules. 2024 Aug 21;29(16):3950. doi: 10.3390/molecules29163950. Molecules. 2024. PMID: 39203027 Free PMC article.
-
Advanced Cellular Models for Rare Disease Study: Exploring Neural, Muscle and Skeletal Organoids.Int J Mol Sci. 2024 Jan 13;25(2):1014. doi: 10.3390/ijms25021014. Int J Mol Sci. 2024. PMID: 38256087 Free PMC article. Review.
-
Tumor Dormancy and Reactivation: The Role of Heat Shock Proteins.Cells. 2024 Jun 23;13(13):1087. doi: 10.3390/cells13131087. Cells. 2024. PMID: 38994941 Free PMC article. Review.
References
-
- Miller KD, Ostrom QT, Kruchko C, et al. Brain and other central nervous system tumor statistics, 2021. CA Cancer J Clin. 2021;71(5):381‐406. - PubMed
-
- Schaff LR, Mellinghoff IK. Glioblastoma and other primary brain malignancies in adults: a review. JAMA. 2023;329(7):574‐587. - PubMed
-
- Cohen AR. Brain tumors in children. N Engl J Med. 2022;386(20):1922‐1931. - PubMed
-
- Bao Z, Wang Y, Wang Q, et al. Intratumor heterogeneity, microenvironment, and mechanisms of drug resistance in glioma recurrence and evolution. Front Med. 2021;15(4):551‐561. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
