Applications of Anti-Cytomegalovirus T Cells for Cancer (Immuno)Therapy
- PMID: 37568582
- PMCID: PMC10416821
- DOI: 10.3390/cancers15153767
Applications of Anti-Cytomegalovirus T Cells for Cancer (Immuno)Therapy
Abstract
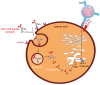
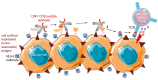
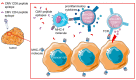
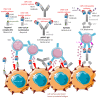
Infection with cytomegalovirus (CMV) is highly prevalent in the general population and largely controlled by CD8pos T cells. Intriguingly, anti-CMV T cells accumulate over time to extraordinarily high numbers, are frequently present as tumor-resident 'bystander' T cells, and remain functional in cancer patients. Consequently, various strategies for redirecting anti-CMV CD8pos T cells to eliminate cancer cells are currently being developed. Here, we provide an overview of these strategies including immunogenic CMV peptide-loading onto endogenous HLA complexes on cancer cells and the use of tumor-directed fusion proteins containing a preassembled CMV peptide/HLA-I complex. Additionally, we discuss conveying the advantageous characteristics of anti-CMV T cells in adoptive cell therapy. Utilization of anti-CMV CD8pos T cells to generate CAR T cells promotes their in vivo persistence and expansion due to appropriate co-stimulation through the endogenous (CMV-)TCR signaling complex. Designing TCR-engineered T cells is more challenging, as the artificial and endogenous TCR compete for expression. Moreover, the use of expanded/reactivated anti-CMV T cells to target CMV peptide-expressing glioblastomas is discussed. This review highlights the most important findings and compares the benefits, disadvantages, and challenges of each strategy. Finally, we discuss how anti-CMV T cell therapies can be further improved to enhance treatment efficacy.
Keywords: ACT; CMV; T cells; cancer immunotherapy; memory inflation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
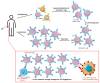
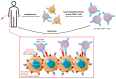
Similar articles
-
Novel Fab-peptide-HLA-I fusion proteins for redirecting pre-existing anti-CMV T cell immunity to selective eliminate carcinoma cells.Oncoimmunology. 2023 May 10;12(1):2207868. doi: 10.1080/2162402X.2023.2207868. eCollection 2023. Oncoimmunology. 2023. PMID: 37180637 Free PMC article.
-
NKG2Cpos NK Cells Regulate the Expansion of Cytomegalovirus-Specific CD8 T Cells.J Immunol. 2020 Jun 1;204(11):2910-2917. doi: 10.4049/jimmunol.1901281. Epub 2020 Apr 13. J Immunol. 2020. PMID: 32284334
-
Refining human T-cell immunotherapy of cytomegalovirus disease: a mouse model with 'humanized' antigen presentation as a new preclinical study tool.Med Microbiol Immunol. 2016 Dec;205(6):549-561. doi: 10.1007/s00430-016-0471-0. Epub 2016 Aug 18. Med Microbiol Immunol. 2016. PMID: 27539576 Review.
-
Soluble recombinant CMVpp65 spanning multiple HLA alleles for reconstitution of antiviral CD4+ and CD8+ T-cell responses after allogeneic stem cell transplantation.J Immunother. 2010 Jan;33(1):60-72. doi: 10.1097/CJI.0b013e3181b56dcc. J Immunother. 2010. PMID: 19952955
-
Cytomegalovirus Infection and Memory T Cell Inflation.Immune Netw. 2015 Aug;15(4):186-90. doi: 10.4110/in.2015.15.4.186. Epub 2015 Aug 26. Immune Netw. 2015. PMID: 26330804 Free PMC article. Review.
Cited by
-
Leveraging oncovirus-derived antigen against the viral malignancies in adoptive cell therapies.Biomark Res. 2024 Jul 29;12(1):71. doi: 10.1186/s40364-024-00617-6. Biomark Res. 2024. PMID: 39075601 Free PMC article. Review.
References
-
- Fowler K., Mucha J., Neumann M., Lewandowski W., Kaczanowska M., Grys M., Schmidt E., Natenshon A., Talarico C., Buck P.O., et al. A systematic literature review of the global seroprevalence of cytomegalovirus: Possible implications for treatment, screening, and vaccine development. BMC Public Health. 2022;22:1659. doi: 10.1186/s12889-022-13971-7. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

