Experimental Induction of Acute Acanthamoeba castellanii Keratitis in Cats
- PMID: 37566398
- PMCID: PMC10424800
- DOI: 10.1167/tvst.12.8.10
Experimental Induction of Acute Acanthamoeba castellanii Keratitis in Cats
Abstract
Purpose: To develop a feline model of acute Acanthamoeba keratitis using methods that replicate natural routes of infection transmission.
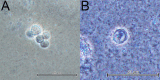
Methods: Corneal Acanthamoeba castellanii inoculation was performed by three methods: topical inoculation with Acanthamoeba solution following corneal abrasion, placement of a contaminated contact lens for 7 days, and placement of a contaminated contact lens for 7 days following corneal abrasion. Sham inoculations with parasite-free medium and sterile contact lenses were also performed. Cats were monitored by ocular examination and in vivo corneal confocal microscopy for 21 days post-inoculation. Corneal samples were collected at intervals for microbiologic assessment, histopathology, and immunohistochemistry.
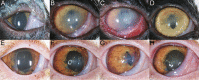
Results: All cats in the corneal abrasion groups developed clinical keratitis. Clinical ocular disease was inconsistently detected in cats from the contaminated contact lens only group. Initial corneal lesions were characterized by multifocal epithelial leukocyte infiltrates. Ocular lesions progressed to corneal epithelial ulceration and diffuse stromal inflammation. After 14 days, corneal ulcerations resolved, and stromal inflammation consolidated into multifocal subepithelial and stromal infiltrates. Corneal amoebae were detected by culture, in vivo confocal microscopy, histopathology, and immunohistochemistry in cats with keratitis. Neutrophilic and lymphocytic keratoconjunctivitis with lymphoplasmacytic anterior uveitis were identified by histopathology. Coinfection with aerobic bacteria was detected in some, but not all, cats with keratitis. Ocular disease was not detected in the sham inoculation groups.
Conclusions: Feline Acanthamoeba keratitis is experimentally transmissible by contaminated contact lenses and topical inoculation following corneal epithelial trauma.
Translational relevance: Experimentally induced acute Acanthamoeba keratitis in cats is clinically and histopathologically similar to its human counterpart.
Conflict of interest statement
Disclosure:
Figures



Similar articles
-
Susceptibility of the Intact and Traumatized Feline Cornea to In Vitro Binding and Invasion by Acanthamoeba castellanii.Cornea. 2023 May 1;42(5):624-629. doi: 10.1097/ICO.0000000000003220. Epub 2022 Dec 13. Cornea. 2023. PMID: 36518074 Free PMC article.
-
Acanthamoeba sclerokeratitis in a cat.J Am Vet Med Assoc. 2020 Dec 15;257(12):1280-1287. doi: 10.2460/javma.257.12.1280. J Am Vet Med Assoc. 2020. PMID: 33269959
-
Detection of free-living amoebae in domestic cats with and without naturally-acquired keratitis.Vet J. 2021 Aug;274:105712. doi: 10.1016/j.tvjl.2021.105712. Epub 2021 Jun 25. Vet J. 2021. PMID: 34182073
-
The diagnosis and management of Acanthamoeba keratitis.CLAO J. 2000 Jan;26(1):47-51. CLAO J. 2000. PMID: 10656311 Review.
-
[Acanthamoeba keratitis--a rare and often late diagnosed disease].Klin Monbl Augenheilkd. 2012 May;229(5):521-8. doi: 10.1055/s-0031-1299539. Epub 2012 May 16. Klin Monbl Augenheilkd. 2012. PMID: 22592343 Review. German.
Cited by
-
The role of naturally acquired intracellular Pseudomonas aeruginosa in the development of Acanthamoeba keratitis in an animal model.PLoS Negl Trop Dis. 2024 Jan 2;18(1):e0011878. doi: 10.1371/journal.pntd.0011878. eCollection 2024 Jan. PLoS Negl Trop Dis. 2024. PMID: 38166139 Free PMC article.
References
-
- Rayamajhee B, Willcox MD, Henriquez FL, Petsoglou C, Carnt N.. Acanthamoeba keratitis: an increasingly common infectious disease of the cornea. Lancet Microbe. 2021; 2(8): e345–e346. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

