The endosomal system of primary human vascular endothelial cells and albumin-FcRn trafficking
- PMID: 37565427
- PMCID: PMC10445748
- DOI: 10.1242/jcs.260912
The endosomal system of primary human vascular endothelial cells and albumin-FcRn trafficking
Abstract
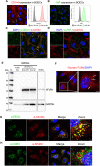
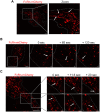
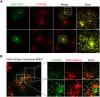
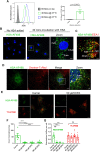
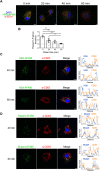
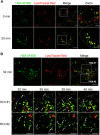
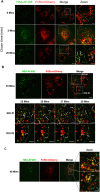
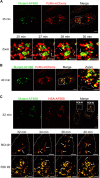
Human serum albumin (HSA) has a long circulatory half-life owing, in part, to interaction with the neonatal Fc receptor (FcRn or FCGRT) in acidic endosomes and recycling of internalised albumin. Vascular endothelial and innate immune cells are considered the most relevant cells for FcRn-mediated albumin homeostasis in vivo. However, little is known about endocytic trafficking of FcRn-albumin complexes in primary human endothelial cells. To investigate FcRn-albumin trafficking in physiologically relevant endothelial cells, we generated primary human vascular endothelial cell lines from blood endothelial precursors, known as blood outgrowth endothelial cells (BOECs). We mapped the endosomal system in BOECs and showed that BOECs efficiently internalise fluorescently labelled HSA predominantly by fluid-phase macropinocytosis. Pulse-chase studies revealed that intracellular HSA molecules co-localised with FcRn in acidic endosomal structures and that the wildtype HSA, but not the non-FcRn-binding HSAH464Q mutant, was excluded from late endosomes and/or lysosomes. Live imaging revealed that HSA is partitioned into FcRn-positive tubules derived from maturing macropinosomes, which are then transported towards the plasma membrane. These findings identify the FcRn-albumin trafficking pathway in primary vascular endothelial cells, relevant to albumin homeostasis.
Keywords: Albumin; Blood outgrowth endothelial cells; Cargo recycling; Macropinocytosis; Neonatal Fc receptor; Primary endothelial cells.
© 2023. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests S.K.D. and A.M.V. are employees of CSL Limited and are able to partake in employee share option schemes. Research funding from CSL Limited is provided through an Australian Research Council linkage grant collaboration.
Figures

Similar articles
-
FcRn mediates fast recycling of endocytosed albumin and IgG from early macropinosomes in primary macrophages.J Cell Sci. 2019 Oct 1;133(5):jcs235416. doi: 10.1242/jcs.235416. J Cell Sci. 2019. PMID: 31444284
-
Direct demonstration of a neonatal Fc receptor (FcRn)-driven endosomal sorting pathway for cellular recycling of albumin.J Biol Chem. 2017 Aug 11;292(32):13312-13322. doi: 10.1074/jbc.M117.794248. Epub 2017 Jun 21. J Biol Chem. 2017. PMID: 28637874 Free PMC article.
-
Dynamics of intracellular neonatal Fc receptor-ligand interactions in primary macrophages using biophysical fluorescence techniques.Mol Biol Cell. 2022 Jan 1;33(1):ar6. doi: 10.1091/mbc.E21-02-0061. Epub 2021 Nov 3. Mol Biol Cell. 2022. PMID: 34731029 Free PMC article.
-
Are endosomal trafficking parameters better targets for improving mAb pharmacokinetics than FcRn binding affinity?Mol Immunol. 2013 Dec;56(4):660-74. doi: 10.1016/j.molimm.2013.05.008. Epub 2013 Aug 2. Mol Immunol. 2013. PMID: 23917469 Review.
-
FcRn expression in cancer: Mechanistic basis and therapeutic opportunities.J Control Release. 2021 Sep 10;337:248-257. doi: 10.1016/j.jconrel.2021.07.007. Epub 2021 Jul 8. J Control Release. 2021. PMID: 34245786 Review.
References
-
- Andersen, J. T., Dalhus, B., Cameron, J., Daba, M. B., Plumridge, A., Evans, L., Brennan, S. O., Gunnarsen, K. S., Bjørås, M., Sleep, D.et al. (2012). Structure-based mutagenesis reveals the albumin-binding site of the neonatal Fc receptor. Nat. Commun. 3, 610. 10.1038/ncomms1607 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

