Three specific gut bacteria in the occurrence and development of colorectal cancer: a concerted effort
- PMID: 37554340
- PMCID: PMC10405800
- DOI: 10.7717/peerj.15777
Three specific gut bacteria in the occurrence and development of colorectal cancer: a concerted effort
Abstract
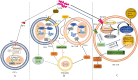
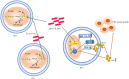
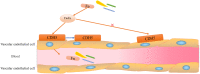
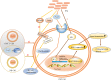
Colorectal cancer (CRC), which develops from the gradual evolution of tubular adenomas and serrated polyps in the colon and rectum, has a poor prognosis and a high mortality rate. In addition to genetics, lifestyle, and chronic diseases, intestinal integrity and microbiota (which facilitate digestion, metabolism, and immune regulation) could promote CRC development. For example, enterotoxigenic Bacteroides fragilis, genotoxic Escherichia coli (pks+ E. coli), and Fusobacterium nucleatum, members of the intestinal microbiota, are highly correlated in CRC. This review describes the roles and mechanisms of these three bacteria in CRC development. Their interaction during CRC initiation and progression has also been proposed. Our view is that in the precancerous stage of colorectal cancer, ETBF causes inflammation, leading to potential changes in intestinal ecology that may provide the basic conditions for pks+ E. coli colonization and induction of oncogenic mutations, when cancerous intestinal epithelial cells can further recruit F. nucleatum to colonise the lesion site and F. nucleatum may contribute to CRC advancement by primarily the development of cancer cells, stemization, and proliferation, which could create new and tailored preventive, screening and therapeutic interventions. However, there is the most dominant microbiota in each stage of CRC development, not neglecting the possibility that two or even all three bacteria could be engaged at any stage of the disease. The relationship between the associated gut microbiota and CRC development may provide important information for therapeutic strategies to assess the potential use of the associated gut microbiota in CRC studies, antibiotic therapy, and prevention strategies.
Keywords: Enterotoxigenic Bacteroides fragilis; Fusobacterium nucleatum; Genotoxic Escherichia coli; Gut microbiota.
© 2023 Gong et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Fusobacterium and Escherichia: models of colorectal cancer driven by microbiota and the utility of microbiota in colorectal cancer screening.Expert Rev Gastroenterol Hepatol. 2015 May;9(5):651-7. doi: 10.1586/17474124.2015.1001745. Epub 2015 Jan 12. Expert Rev Gastroenterol Hepatol. 2015. PMID: 25582922 Review.
-
Aspirin Modulation of the Colorectal Cancer-Associated Microbe Fusobacterium nucleatum.mBio. 2021 Apr 6;12(2):e00547-21. doi: 10.1128/mBio.00547-21. mBio. 2021. PMID: 33824205 Free PMC article.
-
Intratumoral presence of the genotoxic gut bacteria pks+ E. coli, Enterotoxigenic Bacteroides fragilis, and Fusobacterium nucleatum and their association with clinicopathological and molecular features of colorectal cancer.Br J Cancer. 2024 Mar;130(5):728-740. doi: 10.1038/s41416-023-02554-x. Epub 2024 Jan 10. Br J Cancer. 2024. PMID: 38200234 Free PMC article.
-
Diet-mediated gut microbial community modulation and signature metabolites as potential biomarkers for early diagnosis, prognosis, prevention and stage-specific treatment of colorectal cancer.J Adv Res. 2023 Oct;52:45-57. doi: 10.1016/j.jare.2022.12.015. Epub 2022 Dec 31. J Adv Res. 2023. PMID: 36596411 Free PMC article. Review.
-
[The Relationship of Enterotoxigenic Bacteroides fragilis and Fusobacterium nucleatum Intestinal Colonization with Colorectal Cancer: A Case-Control Study Performed with Colon Biopsies].Mikrobiyol Bul. 2023 Jul;57(3):353-364. doi: 10.5578/mb.20239929. Mikrobiyol Bul. 2023. PMID: 37462300 Turkish.
Cited by
-
Association of Polyamine Intake, Other Dietary Components, and Fecal Content of N-acetyl Putrescine and Cadaverine with Patients' Colorectal Lesions.Nutrients. 2024 Aug 29;16(17):2894. doi: 10.3390/nu16172894. Nutrients. 2024. PMID: 39275210 Free PMC article.
-
Application of Fusobacterium nucleatum as a biomarker in gastrointestinal malignancies.World J Gastrointest Oncol. 2024 Jun 15;16(6):2271-2283. doi: 10.4251/wjgo.v16.i6.2271. World J Gastrointest Oncol. 2024. PMID: 38994170 Free PMC article.
-
The histologic features, molecular features, detection and management of serrated polyps: a review.Front Oncol. 2024 Mar 7;14:1356250. doi: 10.3389/fonc.2024.1356250. eCollection 2024. Front Oncol. 2024. PMID: 38515581 Free PMC article. Review.
References
-
- Abed J, Emgard JE, Zamir G, Faroja M, Almogy G, Grenov A, Sol A, Naor R, Pikarsky E, Atlan KA, Mellul A, Chaushu S, Manson AL, Earl AM, Ou N, Brennan CA, Garrett WS, Bachrach G. Fap2 mediates fusobacterium nucleatum colorectal adenocarcinoma enrichment by binding to tumor-expressed Gal-GalNAc. Cell Host & Microbe. 2016;20(2):215–225. doi: 10.1016/j.chom.2016.07.006. - DOI - PMC - PubMed
-
- Bao Y, Tang J, Qian Y, Sun T, Chen H, Chen Z, Sun D, Zhong M, Chen H, Hong J, Chen Y, Fang JY. Long noncoding RNA BFAL1 mediates enterotoxigenic Bacteroides fragilis-related carcinogenesis in colorectal cancer via the RHEB/mTOR pathway. Cell Death & Disease. 2019;10(9):675. doi: 10.1038/s41419-019-1925-2. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

