Antibody-mediated rejection in xenotransplantation: Can it be prevented or reversed?
- PMID: 37548030
- PMCID: PMC11101061
- DOI: 10.1111/xen.12816
Antibody-mediated rejection in xenotransplantation: Can it be prevented or reversed?
Abstract
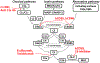
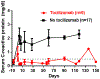
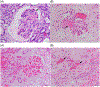
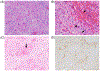
Antibody-mediated rejection (AMR) is the commonest cause of failure of a pig graft after transplantation into an immunosuppressed nonhuman primate (NHP). The incidence of AMR compared to acute cellular rejection is much higher in xenotransplantation (46% vs. 7%) than in allotransplantation (3% vs. 63%) in NHPs. Although AMR in an allograft can often be reversed, to our knowledge there is no report of its successful reversal in a pig xenograft. As there is less experience in preventing or reversing AMR in models of xenotransplantation, the results of studies in patients with allografts provide more information. These include (i) depletion or neutralization of serum anti-donor antibodies, (ii) inhibition of complement activation, (iii) therapies targeting B or plasma cells, and (iv) anti-inflammatory therapy. Depletion or neutralization of anti-pig antibody, for example, by plasmapheresis, is effective in depleting antibodies, but they recover within days. IgG-degrading enzymes do not deplete IgM. Despite the expression of human complement-regulatory proteins on the pig graft, inhibition of systemic complement activation may be necessary, particularly if AMR is to be reversed. Potential therapies include (i) inhibition of complement activation (e.g., by IVIg, C1 INH, or an anti-C5 antibody), but some complement inhibitors are not effective in NHPs, for example, eculizumab. Possible B cell-targeted therapies include (i) B cell depletion, (ii) plasma cell depletion, (iii) modulation of B cell activation, and (iv) enhancing the generation of regulatory B and/or T cells. Among anti-inflammatory agents, anti-IL6R mAb and TNF blockers are increasingly being tested in xenotransplantation models, but with no definitive evidence that they reverse AMR. Increasing attention should be directed toward testing combinations of the above therapies. We suggest that treatment with a systemic complement inhibitor is likely to be most effective, possibly combined with anti-inflammatory agents (if these are not already being administered). Ultimately, it may require further genetic engineering of the organ-source pig to resolve the problem entirely, for example, knockout or knockdown of SLA, and/or expression of PD-L1, HLA E, and/or HLA-G.
Keywords: B cells; anti-pigm; antibodies; antibody-mediated; complement; genetically-engineered; pig; plasma cells; rejection.
© 2023 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Conflict of interest statement
CONFLICT OF INTEREST STATEMENT
D.K.C.C. is a consultant to eGenesis Bio of Cambridge, MA, but the opinions expressed in this article are those of the authors, and do not necessarily reflect those of eGenesis. No other author reports a conflict of interest.
Figures


Similar articles
-
Defining the optimum strategy for identifying adults and children with coeliac disease: systematic review and economic modelling.Health Technol Assess. 2022 Oct;26(44):1-310. doi: 10.3310/ZUCE8371. Health Technol Assess. 2022. PMID: 36321689 Free PMC article.
-
Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review.
-
Interventions to improve mental health and well-being in care-experienced children and young people aged less than 25: the CHIMES systematic review.Public Health Res (Southampt). 2024 Dec;12(14):1-124. doi: 10.3310/MKYP6299. Public Health Res (Southampt). 2024. PMID: 39641478
-
Far Posterior Approach for Rib Fracture Fixation: Surgical Technique and Tips.JBJS Essent Surg Tech. 2024 Dec 6;14(4):e23.00094. doi: 10.2106/JBJS.ST.23.00094. eCollection 2024 Oct-Dec. JBJS Essent Surg Tech. 2024. PMID: 39650795 Free PMC article.
-
The effectiveness of abstinence-based and harm reduction-based interventions in reducing problematic substance use in adults who are experiencing homelessness in high income countries: A systematic review and meta-analysis: A systematic review.Campbell Syst Rev. 2024 Apr 21;20(2):e1396. doi: 10.1002/cl2.1396. eCollection 2024 Jun. Campbell Syst Rev. 2024. PMID: 38645303 Free PMC article. Review.
Cited by
-
Current challenges in xenotransplantation.Curr Opin Organ Transplant. 2024 Jun 1;29(3):205-211. doi: 10.1097/MOT.0000000000001146. Epub 2024 Apr 16. Curr Opin Organ Transplant. 2024. PMID: 38529696 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

