Glucocorticoid receptors regulate central amygdala GABAergic synapses in Marchigian-Sardinian alcohol-preferring rats
- PMID: 37547774
- PMCID: PMC10401345
- DOI: 10.1016/j.ynstr.2023.100547
Glucocorticoid receptors regulate central amygdala GABAergic synapses in Marchigian-Sardinian alcohol-preferring rats
Abstract
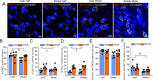
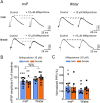
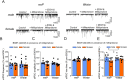
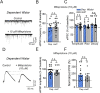
Impairments in the function of the hypothalamic-pituitary-adrenal (HPA) axis and enhanced glucocorticoid receptor (GR) activity in the central amygdala (CeA) are critical mechanisms in the pathogenesis of alcohol use disorder (AUD). The GR antagonist mifepristone attenuates craving in AUD patients, alcohol consumption in AUD models, and decreases CeA γ-aminobutyric acid (GABA) transmission in alcohol-dependent rats. Previous studies suggest elevated GR activity in the CeA of male alcohol-preferring Marchigian-Sardinian (msP) rats, but its contribution to heightened CeA GABA transmission driving their characteristic post-dependent phenotype is largely unknown. We determined Nr3c1 (the gene encoding GR) gene transcription in the CeA in male and female msP and Wistar rats using in situ hybridization and studied acute effects of mifepristone (10 μM) and its interaction with ethanol (44 mM) on pharmacologically isolated spontaneous inhibitory postsynaptic currents (sIPSCs) and electrically evoked inhibitory postsynaptic potentials (eIPSPs) in the CeA using ex vivo slice electrophysiology. Female rats of both genotypes expressed more CeA GRs than males, suggesting a sexually dimorphic GR regulation of CeA activity. Mifepristone reduced sIPSC frequencies (GABA release) and eIPSP amplitudes in msP rats of both sexes, but not in their Wistar counterparts; however, it did not prevent acute ethanol-induced increase in CeA GABA transmission in male rats. In msP rats, GR regulates CeA GABAergic signaling under basal conditions, indicative of intrinsically active GR. Thus, enhanced GR function in the CeA represents a key mechanism contributing to maladaptive behaviors associated with AUD.
© 2023 Published by Elsevier Inc.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



Similar articles
-
Alcohol dependence and withdrawal increase sensitivity of central amygdalar GABAergic synapses to the glucocorticoid receptor antagonist mifepristone in male rats.Neurobiol Dis. 2022 Mar;164:105610. doi: 10.1016/j.nbd.2022.105610. Epub 2022 Jan 4. Neurobiol Dis. 2022. PMID: 34995754 Free PMC article.
-
Enhanced GABAergic transmission in the central nucleus of the amygdala of genetically selected Marchigian Sardinian rats: alcohol and CRF effects.Neuropharmacology. 2013 Apr;67:337-48. doi: 10.1016/j.neuropharm.2012.11.026. Epub 2012 Dec 4. Neuropharmacology. 2013. PMID: 23220399 Free PMC article.
-
Glutamatergic transmission in the central nucleus of the amygdala is selectively altered in Marchigian Sardinian alcohol-preferring rats: Alcohol and CRF effects.Neuropharmacology. 2016 Mar;102:21-31. doi: 10.1016/j.neuropharm.2015.10.027. Epub 2015 Oct 28. Neuropharmacology. 2016. PMID: 26519902 Free PMC article.
-
Genetically selected alcohol-preferring msP rats to study alcohol use disorder: Anything lost in translation?Neuropharmacology. 2021 Mar 15;186:108446. doi: 10.1016/j.neuropharm.2020.108446. Epub 2021 Jan 18. Neuropharmacology. 2021. PMID: 33476639 Free PMC article. Review.
-
Central nucleus of the amygdala and the effects of alcohol and alcohol-drinking behavior in rodents.Pharmacol Biochem Behav. 2002 Mar;71(3):509-15. doi: 10.1016/s0091-3057(01)00680-3. Pharmacol Biochem Behav. 2002. PMID: 11830185 Review.
Cited by
-
Withdrawal from chronic alcohol impairs the serotonin-mediated modulation of GABAergic transmission in the infralimbic cortex in male rats.Neurobiol Dis. 2024 Sep;199:106590. doi: 10.1016/j.nbd.2024.106590. Epub 2024 Jul 10. Neurobiol Dis. 2024. PMID: 38996987
-
Chemogenetic inhibition of central amygdala CRF-expressing neurons decreases alcohol intake but not trauma-related behaviors in a rat model of post-traumatic stress and alcohol use disorder.Mol Psychiatry. 2024 Sep;29(9):2611-2621. doi: 10.1038/s41380-024-02514-8. Epub 2024 Mar 21. Mol Psychiatry. 2024. PMID: 38509197 Free PMC article.
-
Early social isolation differentially affects the glucocorticoid receptor system and alcohol-seeking behavior in male and female Marchigian Sardinian alcohol-preferring rats.Neurobiol Stress. 2023 Dec 7;28:100598. doi: 10.1016/j.ynstr.2023.100598. eCollection 2024 Jan. Neurobiol Stress. 2023. PMID: 38115888 Free PMC article.
-
IL-18 Signaling in the Rat Central Amygdala Is Disrupted in a Comorbid Model of Post-Traumatic Stress and Alcohol Use Disorder.Cells. 2023 Jul 27;12(15):1943. doi: 10.3390/cells12151943. Cells. 2023. PMID: 37566022 Free PMC article.
References
-
- Benvenuti F., Cannella N., Stopponi S., Soverchia L., Ubaldi M., Lunerti V., Vozella V., Cruz B., Roberto M., Ciccocioppo R. Effect of glucocorticoid receptor antagonism on alcohol self-administration in genetically-selected Marchigian Sardinian alcohol-preferring and non-preferring Wistar rats. Int. J. Mol. Sci. 2021;22:4184. doi: 10.3390/ijms22084184. - DOI - PMC - PubMed
-
- Carmack S.A., Vendruscolo J.C.M., Adrienne McGinn M., Miranda-Barrientos J., Repunte-Canonigo V., Bosse G.D., Mercatelli D., Giorgi F.M., Fu Y., Hinrich A.J., Jodelka F.M., Ling K., Messing R.O., Peterson R.T., Rigo F., Edwards S., Sanna P.P., Morales M., Hastings M.L., Koob G.F., Vendruscolo L.F. Corticosteroid sensitization drives opioid addiction. Mol. Psychiatr. 2022;27:2492–2501. doi: 10.1038/s41380-022-01501-1. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

