Fluid retention-associated adverse events in patients treated with BCR::ABL1 inhibitors based on FDA Adverse Event Reporting System (FAERS): a retrospective pharmacovigilance study
- PMID: 37536976
- PMCID: PMC10401248
- DOI: 10.1136/bmjopen-2022-071456
Fluid retention-associated adverse events in patients treated with BCR::ABL1 inhibitors based on FDA Adverse Event Reporting System (FAERS): a retrospective pharmacovigilance study
Abstract
Objectives: This study aimed to conduct a thorough analysis of fluid retention-associated adverse events (AEs) associated with BCR::ABL inhibitors.
Design: A retrospective pharmacovigilance study.
Setting: Food and Drug Administration Adverse Event Reporting System (FAERS) database for BCR::ABL inhibitors was searched from 1 January 2004 to 30 September 2021.
Main outcome measures: Reporting OR (ROR) and 95% CI were used to detect the signals. ROR was calculated by dividing the odds of fluid retention event reporting for the target drug by the odds of fluid retention event reporting for all other drugs. The signal was considered positive if the lower limit of 95% CI of ROR was >1. The analysis was run only considering coupled fluid retention events/BCR::ABL inhibitors with at least three cases.
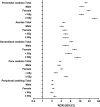
Results: A total of 97 823 reports were identified in FAERS. Imatinib had the most fluid retention signals, followed by dasatinib and nilotinib, while bosutinib and ponatinib had fewer signals. Periorbital oedema (ROR=24.931, 95% CI 22.404 to 27.743), chylothorax (ROR=161.427, 95% CI 125.835 to 207.085), nipple swelling (ROR=48.796, 95% CI 26.270 to 90.636), chylothorax (ROR=35.798, 95% CI 14.791 to 86.642) and gallbladder oedema (ROR=77.996, 95% CI 38.286 to 158.893) were the strongest signals detected for imatinib, dasatinib, nilotinib, bosutinib and ponatinib, respectively. Pleural effusion, pericardial effusion and pulmonary oedema were detected for all BCR::ABL inhibitors, with dasatinib having the highest RORs for pleural effusion (ROR=37.424, 95% CI 35.715 to 39.216), pericardial effusion (ROR=14.146, 95% CI 12.649 to 15.819) and pulmonary oedema (ROR=11.217, 95% CI 10.303 to 12.213). Patients aged ≥65 years using dasatinib, imatinib, nilotinib or bosutinib had higher RORs for pleural effusion, pericardial effusion and pulmonary oedema. Patients aged ≥65 years and females using imatinib had higher RORs for periorbital oedema, generalised oedema and face oedema.
Conclusions: This pharmacovigilance study serves as a clinical reminder to physicians to be more vigilant for fluid retention-associated AEs with BCR::ABL inhibitors.
Keywords: CLINICAL PHARMACOLOGY; Leukaemia; ONCOLOGY.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
Similar articles
-
Pharmacovigilance study of BCR-ABL1 tyrosine kinase inhibitors: a safety analysis of the FDA adverse event reporting system.BMC Pharmacol Toxicol. 2024 Feb 23;25(1):20. doi: 10.1186/s40360-024-00741-x. BMC Pharmacol Toxicol. 2024. PMID: 38395895 Free PMC article.
-
Cardiovascular and pulmonary adverse events in patients treated with BCR-ABL inhibitors: Data from the FDA Adverse Event Reporting System.Am J Hematol. 2015 Apr;90(4):E66-72. doi: 10.1002/ajh.23938. Epub 2015 Jan 30. Am J Hematol. 2015. PMID: 25580915 Free PMC article.
-
Healthcare and economic burden of adverse events among patients with chronic myelogenous leukemia treated with BCR-ABL1 tyrosine kinase inhibitors.J Med Econ. 2017 Jul;20(7):687-691. doi: 10.1080/13696998.2017.1302947. Epub 2017 Mar 12. J Med Econ. 2017. PMID: 28287043
-
Dasatinib-induced chylothorax: report of a case and review of the literature.Invest New Drugs. 2020 Oct;38(5):1627-1632. doi: 10.1007/s10637-020-00932-3. Epub 2020 Apr 4. Invest New Drugs. 2020. PMID: 32248338 Review.
-
Treatment-, patient-, and disease-related factors and the emergence of adverse events with tyrosine kinase inhibitors for the treatment of chronic myeloid leukemia.Pharmacotherapy. 2013 Aug;33(8):868-81. doi: 10.1002/phar.1266. Epub 2013 Apr 3. Pharmacotherapy. 2013. PMID: 23553655 Review.
Cited by
-
Pericardial Disease in Patients with Cancer: Clinical Insights on Diagnosis and Treatment.Cancers (Basel). 2024 Oct 12;16(20):3466. doi: 10.3390/cancers16203466. Cancers (Basel). 2024. PMID: 39456560 Free PMC article. Review.
References
-
- Deininger MW, Goldman JM, Melo JV. The molecular biology of chronic myeloid leukemia. Blood 2000;96:3343–56. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous