Contemporary enterovirus-D68 isolates infect human spinal cord organoids
- PMID: 37535397
- PMCID: PMC10470749
- DOI: 10.1128/mbio.01058-23
Contemporary enterovirus-D68 isolates infect human spinal cord organoids
Abstract
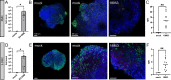
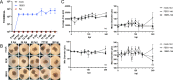
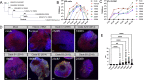
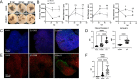
Enterovirus D68 (EV-D68) is a nonpolio enterovirus associated with severe respiratory illness and acute flaccid myelitis (AFM), a polio-like illness causing paralysis in children. AFM outbreaks have been associated with increased circulation and genetic diversity of EV-D68 since 2014, although the virus was discovered in the 1960s. The mechanisms by which EV-D68 targets the central nervous system are unknown. Since enteroviruses are human pathogens that do not routinely infect other animal species, establishment of a human model of the central nervous system is essential for understanding pathogenesis. Here, we describe two human spinal cord organoid (hSCO)-based models for EV-D68 infection derived from induced, pluripotent stem cell (iPSC) lines. One hSCO model consists primarily of spinal motor neurons, while the another model comprises multiple neuronal cell lineages, including motor neurons, interneurons, and glial cells. These hSCOs can be productively infected with contemporary strains, but not a historic strain, of EV-D68 and produce extracellular virus for at least 2 weeks without appreciable cytopathic effect. By comparison, infection with hSCO with another enterovirus, echovirus 11, causes significant structural destruction and apoptosis. Together, these findings suggest that EV-D68 infection is not the sole mediator of neuronal cell death in the spinal cord in those with AFM and that secondary injury from the immune response likely contributes to pathogenesis. IMPORTANCE AFM is a rare condition that causes significant morbidity in affected children, often contributing to life-long sequelae. It is unknown how EV-D68 causes paralysis in children, and effective therapeutic and preventative strategies are not available. Mice are not native hosts for EV-D68, and thus, existing mouse models use immunosuppressed or neonatal mice, mouse-adapted viruses, or intracranial inoculations. To complement existing models, we report two hSCO models for EV-D68 infection. These three-dimensional, multicellular models comprised human cells and include multiple neural lineages, including motor neurons, interneurons, and glial cells. These new hSCO models for EV-D68 infection will contribute to understanding how EV-D68 damages the human spinal cord, which could lead to new therapeutic and prophylactic strategies for this virus.
Keywords: acute flaccid myelitis (AFM); enterovirus; enterovirus D68 (EV-D68); human model systems; induced pluripotent stem cells; organoids; spinal cord organoids.
Conflict of interest statement
J.V.W serves on advisory committees for GlaxoSmithKline and Quidel, which did not impact the work contained in this paper. Other authors are without conflicts.
Figures



Similar articles
-
Contemporary Circulating Enterovirus D68 Strains Infect and Undergo Retrograde Axonal Transport in Spinal Motor Neurons Independent of Sialic Acid.J Virol. 2019 Jul 30;93(16):e00578-19. doi: 10.1128/JVI.00578-19. Print 2019 Aug 15. J Virol. 2019. PMID: 31167912 Free PMC article.
-
Enterovirus D68 Infection in Human Primary Airway and Brain Organoids: No Additional Role for Heparan Sulfate Binding for Neurotropism.Microbiol Spectr. 2022 Oct 26;10(5):e0169422. doi: 10.1128/spectrum.01694-22. Epub 2022 Sep 26. Microbiol Spectr. 2022. PMID: 36154279 Free PMC article.
-
Enterovirus D-68 Infection of Primary Rat Cortical Neurons: Entry, Replication, and Functional Consequences.mBio. 2023 Apr 25;14(2):e0024523. doi: 10.1128/mbio.00245-23. Epub 2023 Mar 6. mBio. 2023. PMID: 36877033 Free PMC article.
-
In vitro and in vivo models for the study of EV-D68 infection.Pathology. 2023 Dec;55(7):907-916. doi: 10.1016/j.pathol.2023.08.007. Epub 2023 Sep 25. Pathology. 2023. PMID: 37852802 Review.
-
Animal Models of Enterovirus D68 Infection and Disease.J Virol. 2022 Aug 10;96(15):e0083322. doi: 10.1128/jvi.00833-22. Epub 2022 Jul 19. J Virol. 2022. PMID: 35852353 Free PMC article. Review.
Cited by
-
Antiviral immunity within neural stem cells distinguishes Enterovirus-D68 strain differences in forebrain organoids.J Neuroinflammation. 2024 Nov 5;21(1):288. doi: 10.1186/s12974-024-03275-5. J Neuroinflammation. 2024. PMID: 39501367 Free PMC article.
-
Inflammatory damage caused by Echovirus 30 in the suckling mouse brain and HMC3 cells.Virol J. 2024 Jul 29;21(1):165. doi: 10.1186/s12985-024-02437-4. Virol J. 2024. PMID: 39075520 Free PMC article.
References
-
- Shah MM, Perez A, Lively JY, Avadhanula V, Boom JA, Chappell J, Englund JA, Fregoe W, Halasa NB, Harrison CJ, Hickey RW, Klein EJ, McNeal MM, Michaels MG, Moffatt ME, Otten C, Sahni LC, Schlaudecker E, Schuster JE, Selvarangan R, Staat MA, Stewart LS, Weinberg GA, Williams JV, Ng TFF, Routh JA, Gerber SI, McMorrow ML, Rha B, Midgley CM. 2021. Enterovirus D68-associated acute respiratory illness ─ new vaccine surveillance network, United States, July-November 2018-2020. MMWR Morb Mortal Wkly Rep 70:1623–1628. doi:10.15585/mmwr.mm7047a1 - DOI - PMC - PubMed
-
- Olsen SJ, Winn AK, Budd AP, Prill MM, Steel J, Midgley CM, Kniss K, Burns E, Rowe T, Foust A, Jasso G, Merced-Morales A, Davis CT, Jang Y, Jones J, Daly P, Gubareva L, Barnes J, Kondor R, Sessions W, Smith C, Wentworth DE, Garg S, Havers FP, Fry AM, Hall AJ, Brammer L, Silk BJ. 2021. Changes in influenza and other respiratory virus activity during the COVID-19 pandemic - United States, 2020-2021. MMWR Morb Mortal Wkly Rep 70:1013–1019. doi:10.15585/mmwr.mm7029a1 - DOI - PMC - PubMed
Publication types
MeSH terms
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
