Synthesis, Characterization, Theoretical and Experimental Anticancer Evaluation of Novel Cocrystals of 5-Fluorouracil and Schiff Bases against SW480 Colorectal Carcinoma
- PMID: 37514115
- PMCID: PMC10383612
- DOI: 10.3390/pharmaceutics15071929
Synthesis, Characterization, Theoretical and Experimental Anticancer Evaluation of Novel Cocrystals of 5-Fluorouracil and Schiff Bases against SW480 Colorectal Carcinoma
Abstract
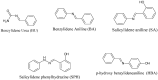
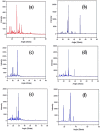
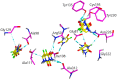
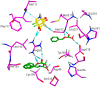
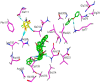
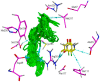
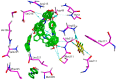
The chemotherapeutic agent known as 5-fluorouracil (5-FU) is an artificial fluoropyrimidine antimetabolite that has been widely used for its antineoplastic properties. Cocrystals of 5-fluorouracil (5-FU) with five different Schiff bases (benzylidene-urea (BU), benzylidene-aniline (BA), salicylidene-aniline (SA), salicylidene-phenylhydrazine (SPH), and para-hydroxy benzylideneaniline (HBA)) are reported in this study. The newly synthesized cocrystals were analyzed by FTIR and PXRD. In this study, we investigated the antitumor efficacy of 5-FU derivatives in SW480 colon cancer cells via MTT assay at varying dose concentrations. Molecular docking was performed to predict the binding mechanism of TS with various 5-FU complexes. FTIR revealed the presence of respective functional groups in the prepared cocrystals. The frequencies (v) of N-H (3220.24 cm-1) and carbonyl groups (1662.38 cm-1) in the spectrum of 5-FU shifted considerably in all derivative cocrystal new interactions. There was a noticeable transformation in the PXRD peak of 5-FU at 2θ = 28.37° in all derivatives. The novelty of the present study lies in the fact that 5-FU-BA showed an anticancer potential IC50 (6.4731) far higher than that of 5-FU (12.116), almost comparable to that of the reference drug doxorubicin (3.3159), against SW480 cancel cell lines, followed by 5-Fu-HBA (10.2174). The inhibition rates of 5-FU-BA and 5-FU-HBA were highest among the derivatives (99.85% and 99.37%, respectively) in comparison with doxorubicin (97.103%). The results revealed that the synthesized 5-FU cocrystals have promising antitumor efficacy compared with previously reported 5-FU and 5-FU. The activities of the cocrystals were rationalized by a molecular modeling approach to envisage binding modes with the target cancer protein.
Keywords: 5-FU; MTT assay; Schiff bases; cocrystallization; computational study.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
[Synthesis, characterization and antitumor activity of 5-fluorouracil-nicotinamide cocrystal].Zhejiang Da Xue Xue Bao Yi Xue Ban. 2017 Mar 25;46(2):127-133. doi: 10.3785/j.issn.1008-9292.2017.04.03. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2017. PMID: 28752703 Free PMC article. Chinese.
-
Cocrystal of 5-Fluorouracil: Characterization and Evaluation of Biopharmaceutical Parameters.AAPS PharmSciTech. 2019 Mar 22;20(4):149. doi: 10.1208/s12249-019-1360-9. AAPS PharmSciTech. 2019. PMID: 30903402
-
Dual antitumor effects of 5-fluorouracil on the cell cycle in colorectal carcinoma cells: a novel target mechanism concept for pharmacokinetic modulating chemotherapy.Cancer Res. 2001 Feb 1;61(3):1029-37. Cancer Res. 2001. PMID: 11221829
-
Schiff Bases and their Metal Complexes as Potential Anticancer Candidates: A Review of Recent Works.Anticancer Agents Med Chem. 2019;19(15):1786-1795. doi: 10.2174/1871520619666190227171716. Anticancer Agents Med Chem. 2019. PMID: 30827264 Review.
-
[Combination of 5-Fluorouracil and folinic acid--is it still the standard therapy for advanced colorectal carcinoma?].Tumori. 2000 Sep-Oct;86(5 Suppl 2):S19-25. Tumori. 2000. PMID: 11195298 Review. Italian.
Cited by
-
Specifics of Pharmacokinetics and Biodistribution of 5-Fluorouracil Polymeric Complex.Molecules. 2023 Dec 15;28(24):8096. doi: 10.3390/molecules28248096. Molecules. 2023. PMID: 38138585 Free PMC article.
References
-
- Sher F., Iqbal S.Z., Jubeen F. Future of 5-fluorouracil in cancer therapeutics, current pharmacokinetics issues and a way forward. J. Cancer Res. Pract. 2019;6:155. doi: 10.4103/JCRP.JCRP_10_19. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

