Src-Dependent NM2A Tyrosine Phosphorylation Regulates Actomyosin Remodeling
- PMID: 37508535
- PMCID: PMC10377941
- DOI: 10.3390/cells12141871
Src-Dependent NM2A Tyrosine Phosphorylation Regulates Actomyosin Remodeling
Abstract
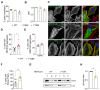
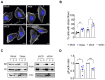
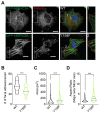
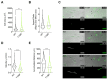
Non-muscle myosin 2A (NM2A) is a key cytoskeletal enzyme that, along with actin, assembles into actomyosin filaments inside cells. NM2A is fundamental for cell adhesion and motility, playing important functions in different stages of development and during the progression of viral and bacterial infections. Phosphorylation events regulate the activity and the cellular localization of NM2A. We previously identified the tyrosine phosphorylation of residue 158 (pTyr158) in the motor domain of the NM2A heavy chain. This phosphorylation can be promoted by Listeria monocytogenes infection of epithelial cells and is dependent on Src kinase; however, its molecular role is unknown. Here, we show that the status of pTyr158 defines cytoskeletal organization, affects the assembly/disassembly of focal adhesions, and interferes with cell migration. Cells overexpressing a non-phosphorylatable NM2A variant or expressing reduced levels of Src kinase display increased stress fibers and larger focal adhesions, suggesting an altered contraction status consistent with the increased NM2A activity that we also observed. We propose NM2A pTyr158 as a novel layer of regulation of actomyosin cytoskeleton organization.
Keywords: NM2A; Src kinase; actomyosin cytoskeleton; cell migration; cytoskeletal remodeling.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
Similar articles
-
Non-Muscle Myosin 2A (NM2A): Structure, Regulation and Function.Cells. 2020 Jul 1;9(7):1590. doi: 10.3390/cells9071590. Cells. 2020. PMID: 32630196 Free PMC article. Review.
-
Tyrosine phosphorylation of cortactin by the FAK-Src complex at focal adhesions regulates cell motility.BMC Cell Biol. 2011 Nov 13;12:49. doi: 10.1186/1471-2121-12-49. BMC Cell Biol. 2011. PMID: 22078467 Free PMC article.
-
The Arf GTPase-activating Protein, ASAP1, Binds Nonmuscle Myosin 2A to Control Remodeling of the Actomyosin Network.J Biol Chem. 2016 Apr 1;291(14):7517-26. doi: 10.1074/jbc.M115.701292. Epub 2016 Feb 17. J Biol Chem. 2016. PMID: 26893376 Free PMC article.
-
Multiple S100 protein isoforms and C-terminal phosphorylation contribute to the paralog-selective regulation of nonmuscle myosin 2 filaments.J Biol Chem. 2018 Sep 21;293(38):14850-14867. doi: 10.1074/jbc.RA118.004277. Epub 2018 Aug 7. J Biol Chem. 2018. PMID: 30087119 Free PMC article.
-
Caldesmon phosphorylation in actin cytoskeletal remodeling.Eur J Cell Biol. 2006 Apr;85(3-4):305-9. doi: 10.1016/j.ejcb.2005.08.008. Epub 2005 Sep 12. Eur J Cell Biol. 2006. PMID: 16546574 Review.
Cited by
-
Exploring the nexus between MYH9 and tumors: novel insights and new therapeutic opportunities.Front Cell Dev Biol. 2024 Aug 1;12:1421763. doi: 10.3389/fcell.2024.1421763. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39149512 Free PMC article. Review.
References
-
- Kendrick-Jones J., Taylor K.A., Scholey J.M. Phosphorylation of nonmuscle myosin and stabilization of thick filament structure. Methods Enzym. 1982;85:364–370. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

